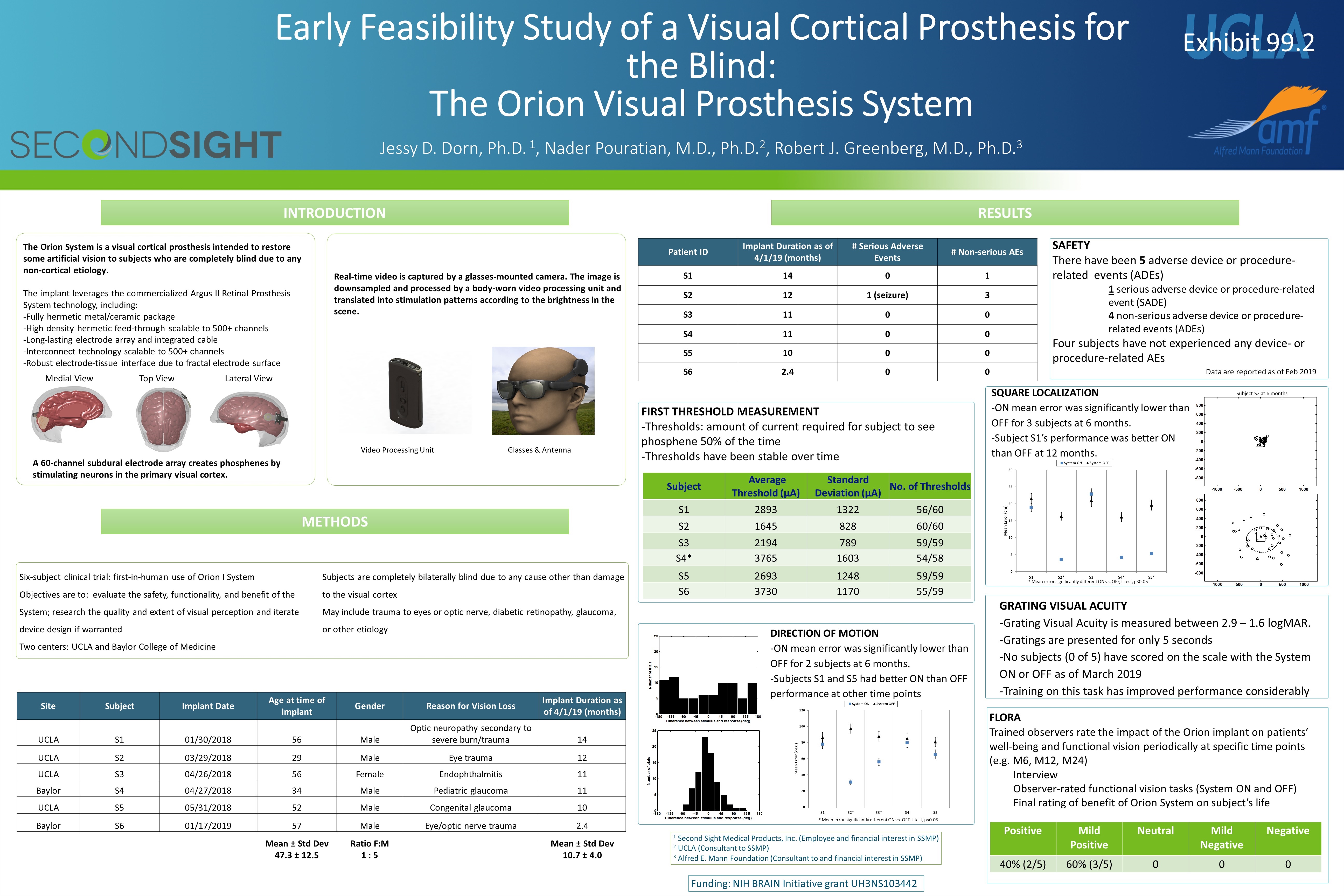

Early Feasibility Study of a Visual Cortical Prosthesis for the Blind: The Orion Visual Prosthesis System Jessy D. Dorn, Ph.D. 1, Nader Pouratian, M.D., Ph.D.2, Robert J. Greenberg, M.D., Ph.D.3 INTRODUCTION METHODS Six-subject clinical trial: first-in-human use of Orion I System Objectives are to: evaluate the safety, functionality, and benefit of the System; research the quality and extent of visual perception and iterate device design if warranted Two centers: UCLA and Baylor College of Medicine Subjects are completely bilaterally blind due to any cause other than damage to the visual cortex May include trauma to eyes or optic nerve, diabetic retinopathy, glaucoma, or other etiology Medial View Top View Lateral View Video Processing Unit Glasses & Antenna SAFETY There have been 5 adverse device or procedure-related events (ADEs) 1 serious adverse device or procedure-related event (SADE) 4 non-serious adverse device or procedure-related events (ADEs) Four subjects have not experienced any device- or procedure-related AEs RESULTS FIRST THRESHOLD MEASUREMENT -Thresholds: amount of current required for subject to see phosphene 50% of the time -Thresholds have been stable over time Data are reported as of Feb 2019 The Orion System is a visual cortical prosthesis intended to restore some artificial vision to subjects who are completely blind due to any non-cortical etiology. The implant leverages the commercialized Argus II Retinal Prosthesis System technology, including: -Fully hermetic metal/ceramic package -High density hermetic feed-through scalable to 500+ channels -Long-lasting electrode array and integrated cable -Interconnect technology scalable to 500+ channels -Robust electrode-tissue interface due to fractal electrode surface Site Subject Implant Date Age at time of implant Gender Reason for Vision Loss Implant Duration as of 4/1/19 (months) UCLA S1 01/30/2018 56 Male Optic neuropathy secondary to severe burn/trauma 14 UCLA S2 03/29/2018 29 Male Eye trauma 12 UCLA S3 04/26/2018 56 Female Endophthalmitis 11 Baylor S4 04/27/2018 34 Male Pediatric glaucoma 11 UCLA S5 05/31/2018 52 Male Congenital glaucoma 10 Baylor S6 01/17/2019 57 Male Eye/optic nerve trauma 2.4 Mean ± Std Dev 47.3 ± 12.5 Ratio F:M 1 : 5 Mean ± Std Dev 10.7 ± 4.0 Patient ID Implant Duration as of 4/1/19 (months) # Serious Adverse Events # Non-serious AEs S1 14 0 1 S2 12 1 (seizure) 3 S3 11 0 0 S4 11 0 0 S5 10 0 0 S6 2.4 0 0 Subject Average Threshold (µA) Standard Deviation (µA) No. of Thresholds S1 2893 1322 56/60 S2 1645 828 60/60 S3 2194 789 59/59 S4* 3765 1603 54/58 S5 2693 1248 59/59 S6 3730 1170 55/59 Subject S2 at 6 months * Mean error significantly different ON vs. OFF, t-test, p<0.05 SQUARE LOCALIZATION -ON mean error was significantly lower than OFF for 3 subjects at 6 months. -Subject S1’s performance was better ON than OFF at 12 months. DIRECTION OF MOTION -ON mean error was significantly lower than OFF for 2 subjects at 6 months. -Subjects S1 and S5 had better ON than OFF performance at other time points * Mean error significantly different ON vs. OFF, t-test, p<0.05 GRATING VISUAL ACUITY -Grating Visual Acuity is measured between 2.9 – 1.6 logMAR. -Gratings are presented for only 5 seconds -No subjects (0 of 5) have scored on the scale with the System ON or OFF as of March 2019 -Training on this task has improved performance considerably FLORA Trained observers rate the impact of the Orion implant on patients’ well-being and functional vision periodically at specific time points (e.g. M6, M12, M24) Interview Observer-rated functional vision tasks (System ON and OFF) Final rating of benefit of Orion System on subject’s life Positive Mild Positive Neutral Mild Negative Negative 40% (2/5) 60% (3/5) 0 0 0 Real-time video is captured by a glasses-mounted camera. The image is downsampled and processed by a body-worn video processing unit and translated into stimulation patterns according to the brightness in the scene. A 60-channel subdural electrode array creates phosphenes by stimulating neurons in the primary visual cortex. Funding: NIH BRAIN Initiative grant UH3NS103442 1 Second Sight Medical Products, Inc. (Employee and financial interest in SSMP) 2 UCLA (Consultant to SSMP) 3 Alfred E. Mann Foundation (Consultant to and financial interest in SSMP) Exhibit 99.2