
Early Feasibility Study of a Visual Cortical Prosthesis for the Blind: The Orion Visual Prosthesis System Jessy D. Dorn, Ph.D. Nader Pouratian, M.D., Ph.D. Robert J. Greenberg, M.D., Ph.D. UH3NS103442 Exhibit 99.3

Disclosures Jessy D. Dorn, Ph.D. – Employee of & financial interest in Second Sight Nader Pouratian, M.D., Ph.D. – Consultant to Second Sight Robert J. Greenberg, M.D., Ph.D. – Consultant to & financial interest in Second Sight Study partly funded by NIH BRAIN Initiative grant: UH3NS103442

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are intended to be covered by the “safe harbor” created by those sections. All statements in this release that are not based on historical fact are “forward looking statements.” These statements may be identified by words such as “estimates,” “anticipates,” “projects,” “plans,” “goal,” or “planned,” “seeks,” “may,” “will,” “expects,” “intends,” “believes,” “should,” and similar expressions, or the negative versions thereof, and which also may be identified by their context. All statements that address operating performance or events or developments that Second Sight expects or anticipates will occur in the future, such as stated objectives or goals, or that are not otherwise historical facts, are forward-looking statements. While management has based any forward-looking statements included in this presentation on its current expectations, the information on which such expectations were based may change. Forward-looking statements involve inherent risks and uncertainties which could cause actual results to differ materially from those in the forward-looking statements as a result of various factors, including those risks and uncertainties described in the Risk Factors and in Management’s Discussion and Analysis of Financial Condition and Results of Operations sections of our Annual Report, on Form 10-K, filed on March 19, 2019, and our other reports filed from time to time with the Securities and Exchange Commission. We urge you to consider those risks and uncertainties in evaluating our forward-looking statements. We caution readers not to place undue reliance upon any such forward-looking statements, which speak only as of the date made. Except as otherwise required by the federal securities laws, we disclaim any obligation or undertaking to publicly release any updates or revisions to any forward-looking statement contained herein (or elsewhere) to reflect any change in our expectations with regard thereto, or any change in events, conditions, or circumstances on which any such statement is based
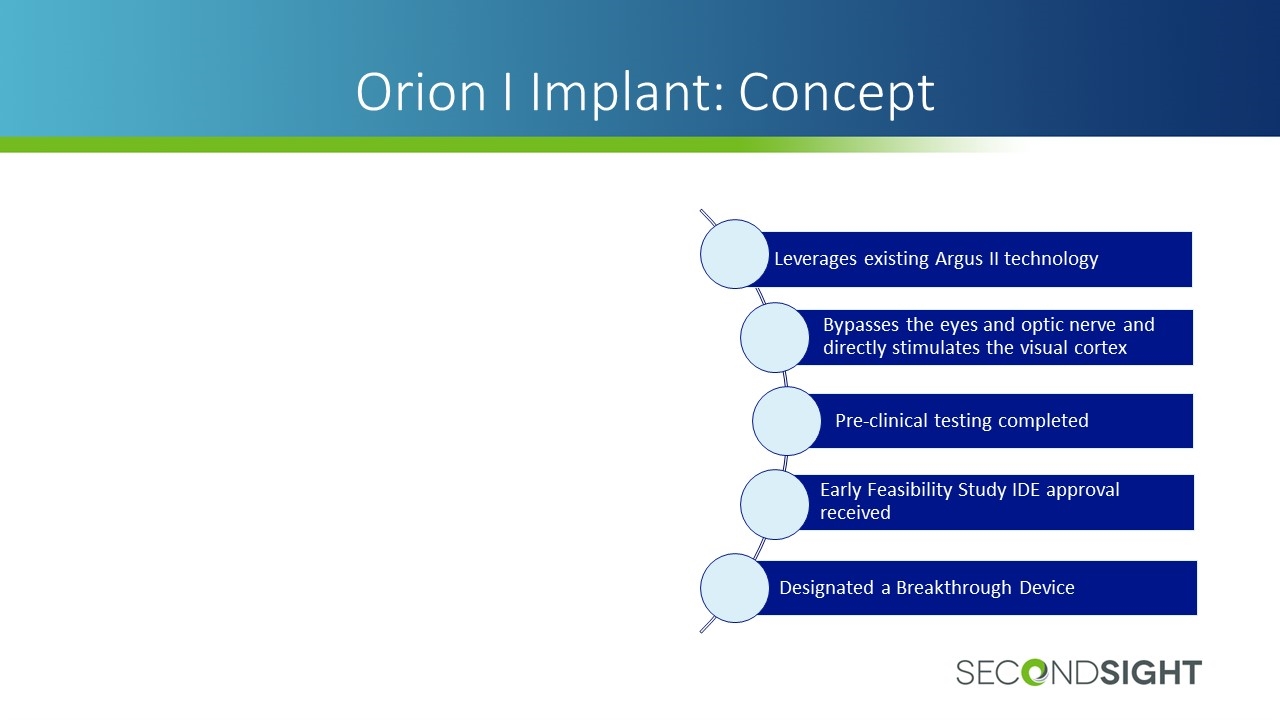
Orion I Implant: Concept Leverages existing Argus II technology Bypasses the eyes and optic nerve and directly stimulates the visual cortex Early Feasibility Study IDE approval received Pre-clinical testing completed Designated a Breakthrough Device
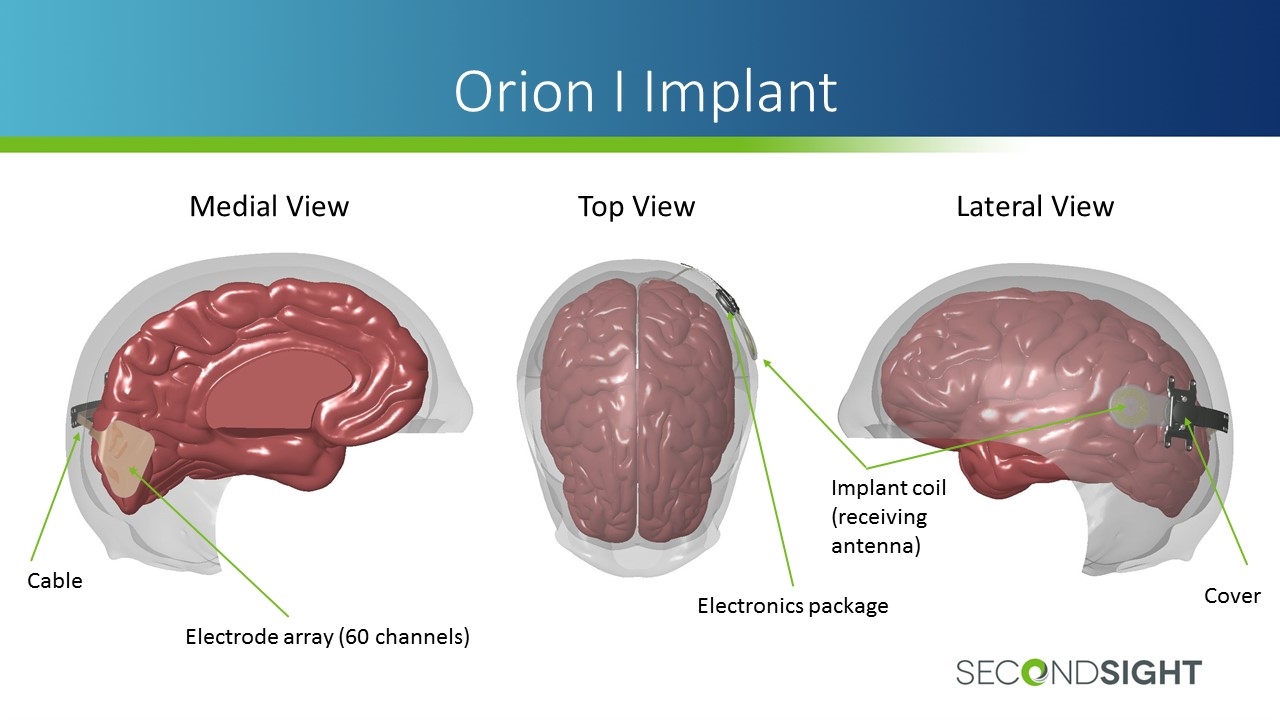
Orion I Implant Medial View Top View Lateral View Electrode array (60 channels) Cable Electronics package Implant coil (receiving antenna) Cover

Orion System: User-Worn Externals Video Processing Unit Glasses & Antenna Real-time video captured by the camera is downsampled and processed by the VPU and converted into stimulation patterns based on the brightness in the scene. Data are power are send wirelessly through RF link to the implant.

Orion Early Feasibility Study Six subjects to be followed for five years Two centers: UCLA and Baylor College of Medicine Primary outcome measure: safety (adverse events) Secondary outcome measures: ability to produce phosphenes, long-term functionality of the device, and benefit in terms of visual function, functional vision, quality of life Subjects are completely bilaterally blind due to any cause other than damage to the visual cortex May include trauma to eyes or optic nerve, diabetic retinopathy, glaucoma, or other etiology
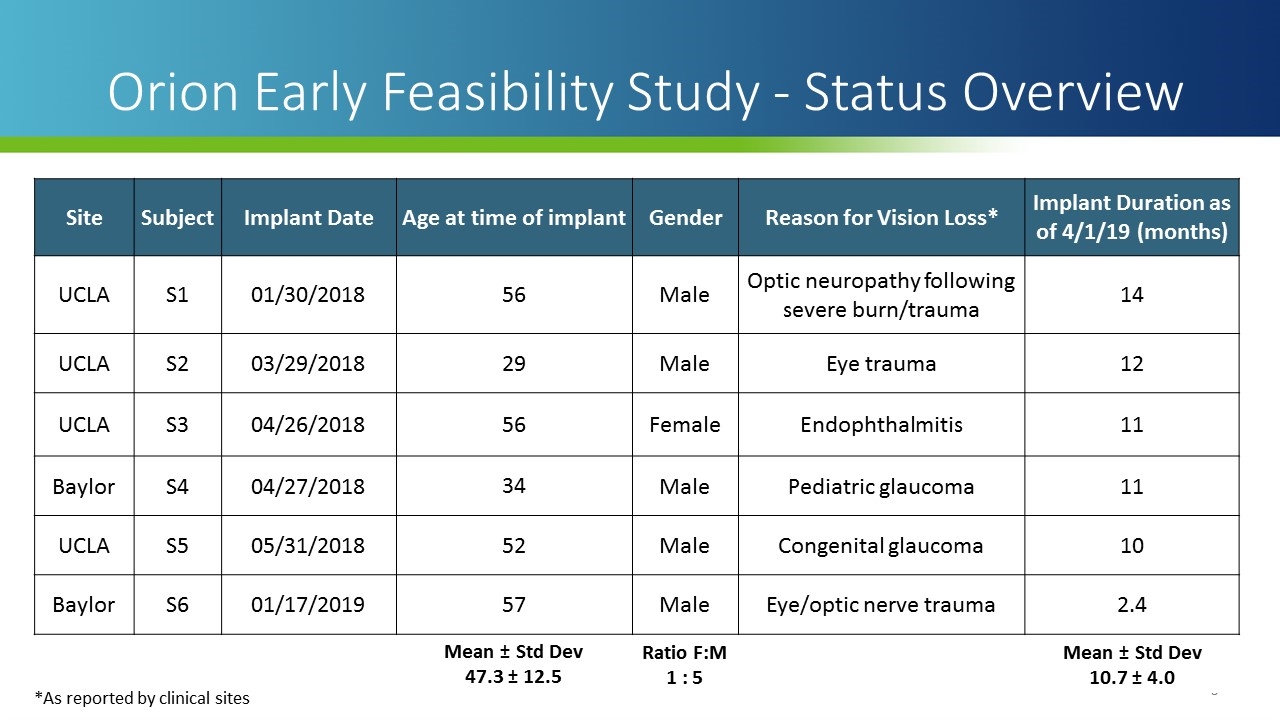
Site Subject Implant Date Age at time of implant Gender Reason for Vision Loss* Implant Duration as of 4/1/19 (months) UCLA S1 01/30/2018 56 Male Optic neuropathy following severe burn/trauma 14 UCLA S2 03/29/2018 29 Male Eye trauma 12 UCLA S3 04/26/2018 56 Female Endophthalmitis 11 Baylor S4 04/27/2018 34 Male Pediatric glaucoma 11 UCLA S5 05/31/2018 52 Male Congenital glaucoma 10 Baylor S6 01/17/2019 57 Male Eye/optic nerve trauma 2.4 Mean ± Std Dev 47.3 ± 12.5 Ratio F:M 1 : 5 Mean ± Std Dev 10.7 ± 4.0 Orion Early Feasibility Study - Status Overview *As reported by clinical sites
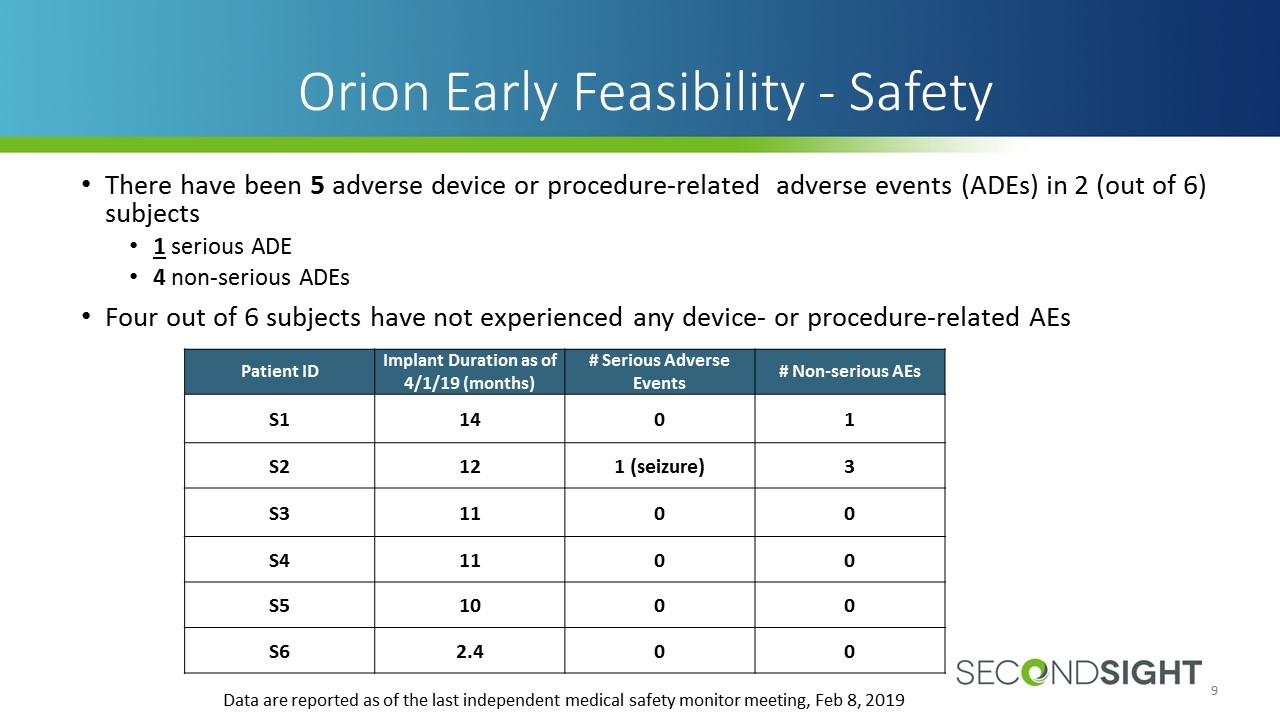
There have been 5 adverse device or procedure-related adverse events (ADEs) in 2 (out of 6) subjects 1 serious ADE 4 non-serious ADEs Four out of 6 subjects have not experienced any device- or procedure-related AEs Orion Early Feasibility - Safety Data are reported as of the last independent medical safety monitor meeting, Feb 8, 2019 Patient ID Implant Duration as of 4/1/19 (months) # Serious Adverse Events # Non-serious AEs S1 14 0 1 S2 12 1 (seizure) 3 S3 11 0 0 S4 11 0 0 S5 10 0 0 S6 2.4 0 0

Research Focus: First 6 Months Create video settings and establish stimulation safety Custom-programming: measure thresholds, perform spatial mapping, create video configuration file – direct stimulation through computer control Testing for comfort and safety during camera-based stimulation Clear subjects for home use Allowing subjects to take the system home quickly was a high priority First five took the device home between 4 and 7 months post-implant Initiate rehabilitation sessions and train subjects on hand-camera coordination, eye movement, and visual skills In-home rehabilitation in progress for five subjects
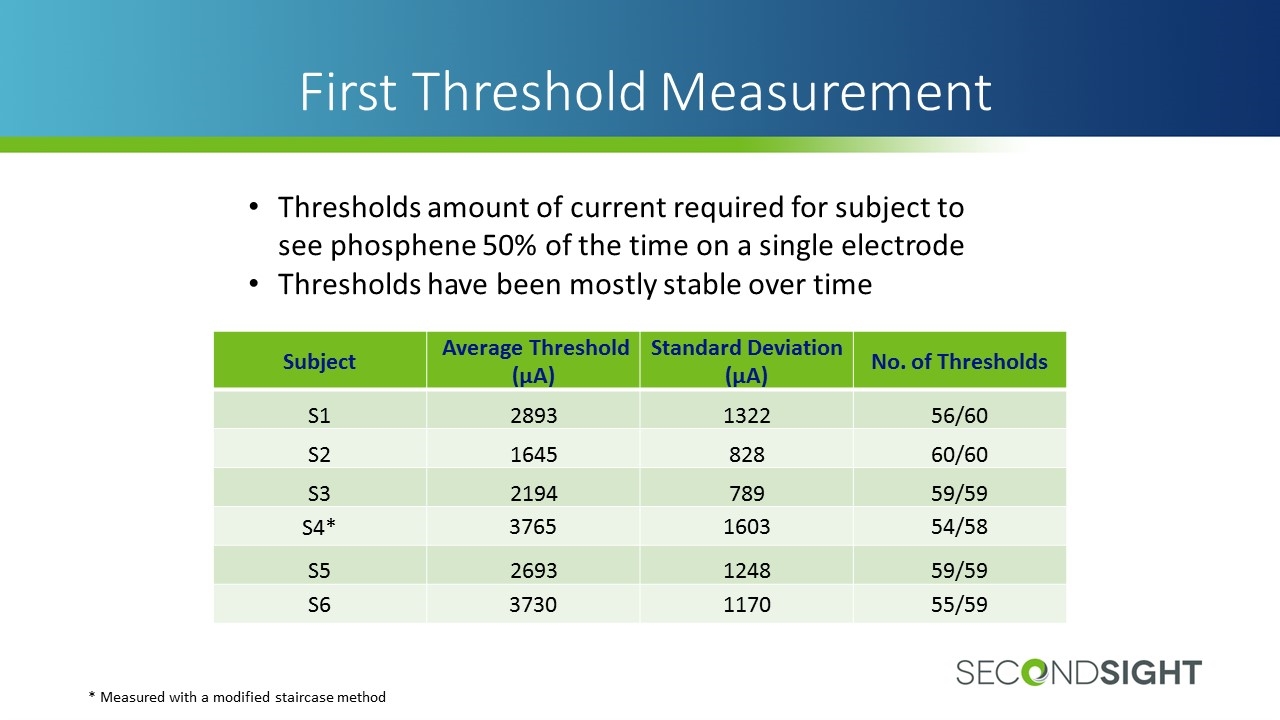
First Threshold Measurement Subject Average Threshold (µA) Standard Deviation (µA) No. of Thresholds S1 2893 1322 56/60 S2 1645 828 60/60 S3 2194 789 59/59 S4* 3765 1603 54/58 S5 2693 1248 59/59 S6 3730 1170 55/59 Thresholds amount of current required for subject to see phosphene 50% of the time on a single electrode Thresholds have been mostly stable over time * Measured with a modified staircase method
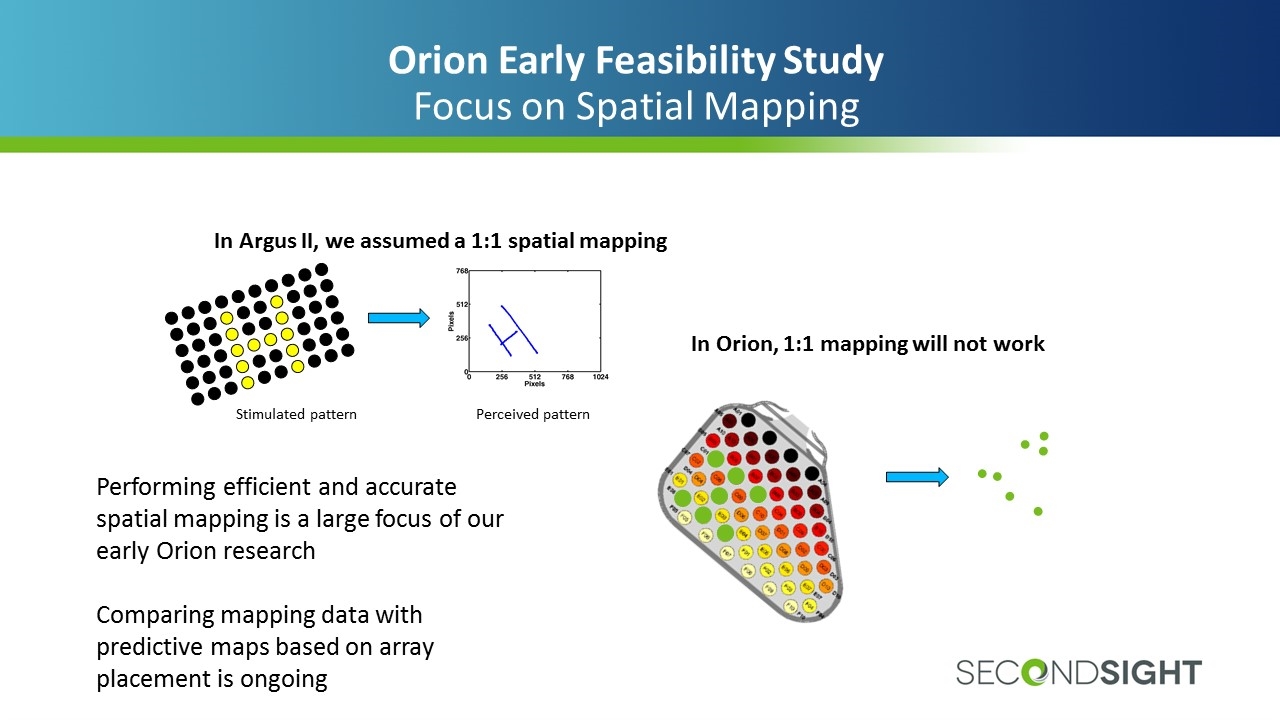
Orion Early Feasibility Study Focus on Spatial Mapping In Argus II, we assumed a 1:1 spatial mapping Stimulated pattern Perceived pattern In Orion, 1:1 mapping will not work Performing efficient and accurate spatial mapping is a large focus of our early Orion research Comparing mapping data with predictive maps based on array placement is ongoing

Orion Study – Performance Measures A suite of assessments to measure performance administered at multiple time points Visual function – objective, controlled, artificial Functional vision – subjective, real-world, more meaningful Well-being & quality of life – patient-reported
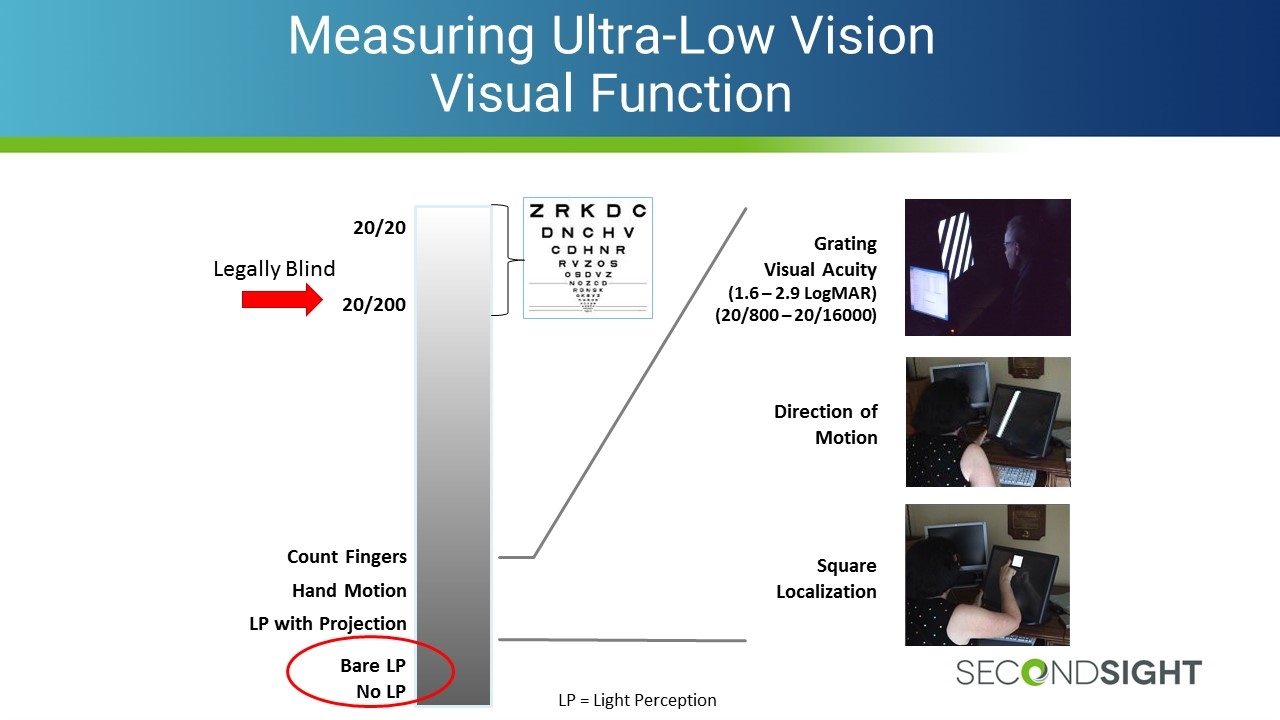
Measuring Ultra-Low Vision Visual Function 20/20 20/200 Bare LP No LP Count Fingers Hand Motion LP with Projection LP = Light Perception Legally Blind Square Localization Direction of Motion Grating Visual Acuity (1.6 – 2.9 LogMAR) (20/800 – 20/16000)
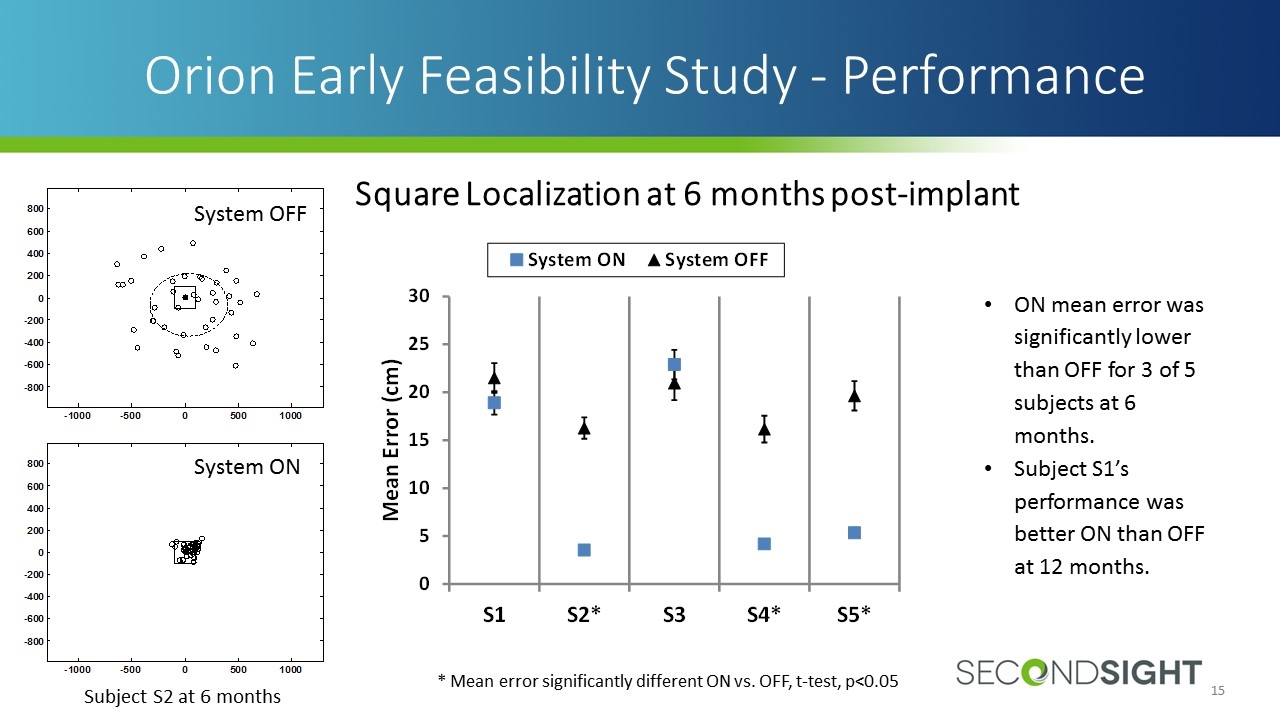
ON mean error was significantly lower than OFF for 3 of 5 subjects at 6 months. Subject S1’s performance was better ON than OFF at 12 months. Orion Early Feasibility Study - Performance * Mean error significantly different ON vs. OFF, t-test, p<0.05 System OFF System ON Subject S2 at 6 months Square Localization at 6 months post-implant
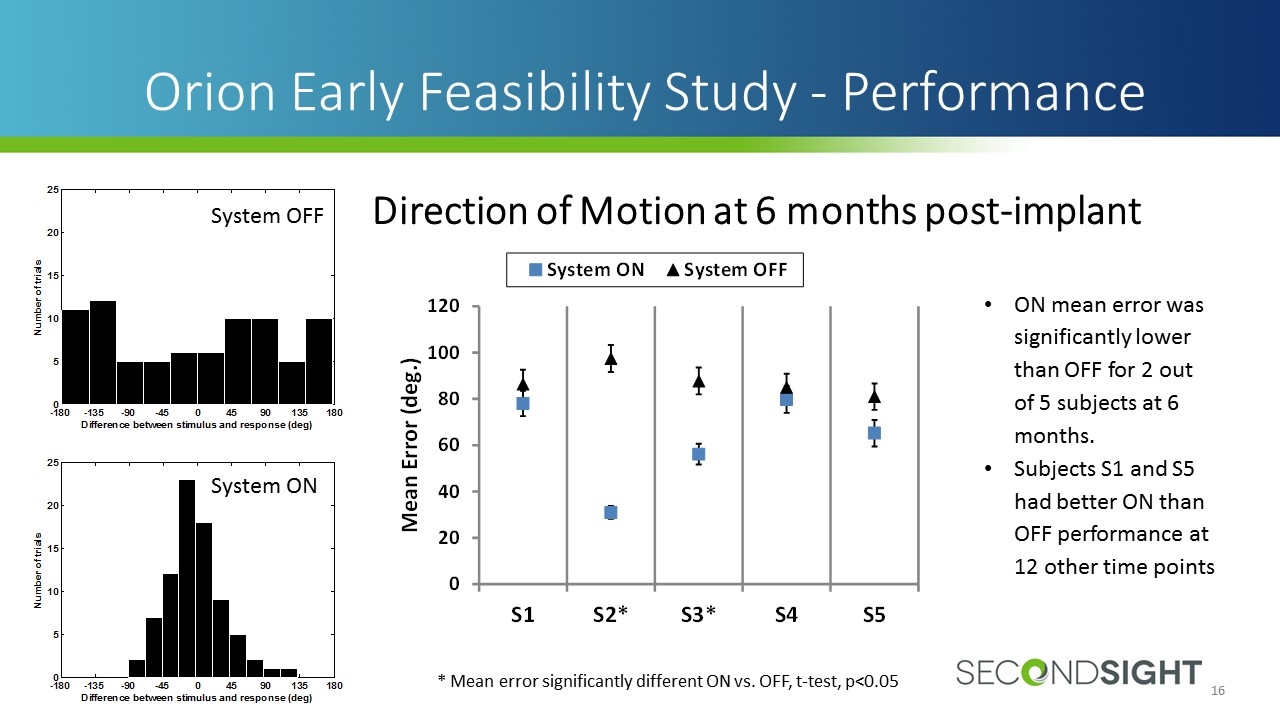
Orion Early Feasibility Study - Performance ON mean error was significantly lower than OFF for 2 out of 5 subjects at 6 months. Subjects S1 and S5 had better ON than OFF performance at 12 other time points * Mean error significantly different ON vs. OFF, t-test, p<0.05 System OFF System ON Direction of Motion at 6 months post-implant
Grating Visual Acuity Grating Visual Acuity is measured between 2.9 – 1.6 logMAR. Gratings are presented for only 5 seconds No subjects (0 of 5) scored on the scale with the System ON or OFF at 6 months post-implant Training on this task has improved performance considerably Orion Early Feasibility Study - Performance
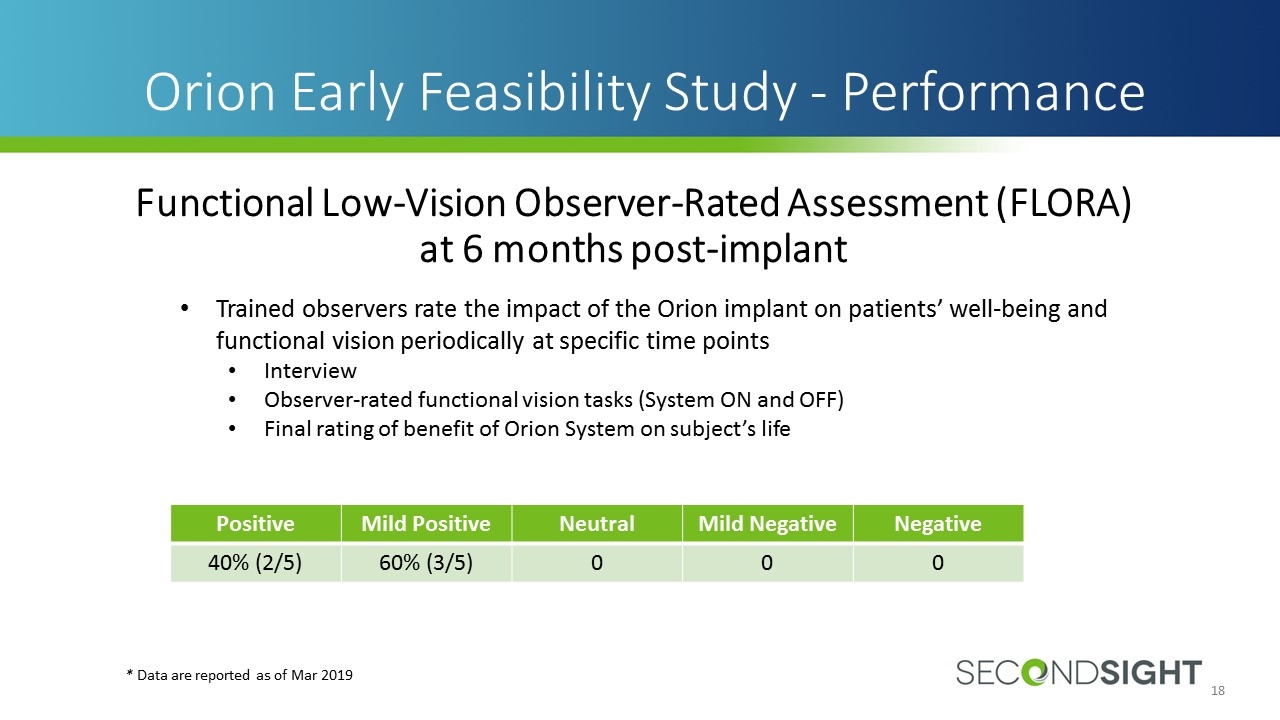
Trained observers rate the impact of the Orion implant on patients’ well-being and functional vision periodically at specific time points Interview Observer-rated functional vision tasks (System ON and OFF) Final rating of benefit of Orion System on subject’s life Orion Early Feasibility Study - Performance Functional Low-Vision Observer-Rated Assessment (FLORA) at 6 months post-implant * Data are reported as of Mar 2019 Positive Mild Positive Neutral Mild Negative Negative 40% (2/5) 60% (3/5) 0 0 0

“Real World Use”: observations from rehabilitation sessions Subjects are finding success with Orion for everyday visual tasks “…He was able to find the cue ball with no problems on the table. He was able to tell the cue ball from the blue ten, and also from balls with a stripe vs. the cue ball. He could find the racked balls at the other end of the table too.” “…we've found that looking forward is where [the Orion] shines better for the user as they can detect upcoming objects... He was able to see cars parked on the side of the street, openings in the sidewalk up into driveways, etc.” “…He was able to order patterns from small checkers, big checkers, and white cloth. There's a half inch difference in checker size in the patterns.” “When working throughout her apartment building, she was able to tell where I was located when standing in front of a 10’ wide light wall w/out visual clutter. She was also able to correctly determine whether I was traveling from left to right or right to left along this wall, 7/10 times.”

Questions? Thank you!