

Second Sight Medical Products, Inc. (NASDAQ: EYES) Discover Life in a New Light® B. Riley FBR Investor Conference May 23, 2019 Ex 99.1

This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange and Exchange Act of 1934, as amended, which are intended to be covered by the "safe harbor" created by those sections. All statements in this release that are not based on historical fact are "forward looking statements." These statements may be identified by words such as "estimates," "anticipates," "projects," "plans" or "planned," "strategy," “goal," "seeks," "may," "will," "expects," "intends," "believes," "should," and similar expressions, or the negative versions thereof, and which also may be identified by their context. All statements that address operating performance or events or developments that Second Sight expects or anticipates will occur in the future, such as stated objectives or goals, or that are not otherwise historical facts, are forward-looking statements. While management has based any forward-looking statements included in this release on its current expectations, the information on which such expectations were based may change. Forward-looking statements involve inherent risks and uncertainties which could cause actual results to differ materially from those in the forward-looking statements as a result of various factors, including those risks and uncertainties described in the Risk Factors and in Management's Discussion and Analysis of Financial Condition and Results of Operations sections of our Annual Report, on Form 10-K, filed on March 19, 2019, our most recent 10-Q, filed on May 15, 2019 and our other reports filed from time to time with the Securities and Exchange Commission. We urge you to consider those risks and uncertainties in evaluating our forward-looking statements. We caution readers not to place undue reliance upon any such forward-looking statements, which speak only as of the date made. Except as otherwise required by the federal securities laws, we disclaim any obligation or undertaking to publicly release any updates or revisions to any forward-looking statement contained herein (or elsewhere) to reflect any change in our expectations with regard thereto, or any change in events, conditions, or circumstances on which any such statement is based. Forward Looking Statements
Two Breakthrough Technologies Argus® II Retinal Prosthesis Legacy Technology Development Platform First and only FDA approved retinal prosthesis 20+ years and ~$200M invested to develop underlying technology and commercialize Durable array design (10+ years) and proprietary software algorithms with 300+ implants Approved for individuals with advanced retinitis pigmentosa (bare-light and no-light perception) in U.S. Transformational technology with U.S. human trial initiated in Q1 2018 Larger potential addressable market with a significant unmet need; includes glaucoma, diabetic retinopathy, optic nerve disease and eye injury/trauma Bypasses the retina and optic nerve to directly stimulate the portion of the brain responsible for vision Orion® Visual Cortical Prosthesis Platform for Broad Commercialization
Orion is a investigational visual cortical prosthesis that induces visual perception in blind individuals Orion Visual Cortical Prosthesis Overview Video Link: https://youtu.be/yiaKNmUIcqs
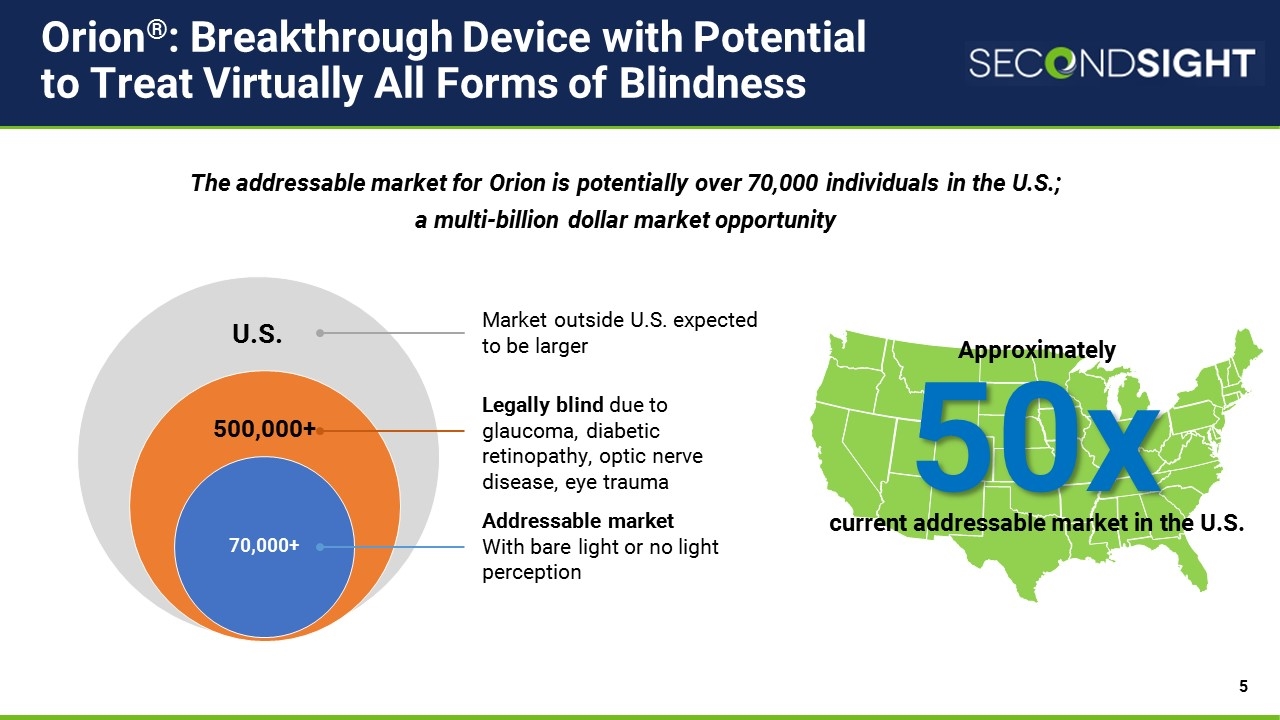
Approximately current addressable market in the U.S. Orion®: Breakthrough Device with Potential to Treat Virtually All Forms of Blindness U.S. 70,000+ 500,000+ Addressable market With bare light or no light perception Legally blind due to glaucoma, diabetic retinopathy, optic nerve disease, eye trauma The addressable market for Orion is potentially over 70,000 individuals in the U.S.; a multi-billion dollar market opportunity 50x Market outside U.S. expected to be larger
Encouraging Orion® Interim Study Results Conducting a six subject feasibility study at Ronald Reagan UCLA Medical Center and Baylor College of Medicine Six subjects cleared for home use Initial results from our testing include the following: Stimulation parameters are within expected ranges and field of view appears to be larger than Argus Observations from rehab sessions indicate subjects are able to do things with Orion that they could not do without, examples include: Locate people in front of them Walk down sidewalk independently and identify parked cars and driveways Identify cue ball and striped balls on a pool table Sort light from dark laundry Identify and blow out candles on a birthday cake Currently, system on versus system off: Square localization: 4 of 5 subjects significantly better Direction of motion: 4 of 5 subjects significantly better FLORA: 5 of 5 subjects are showing benefit or mild benefit Performance data from feasibility subjects will be used to determine next steps in 2019, including discussions with FDA to finalize clinical and regulatory pathway as part of Breakthrough Devices program

Growth Strategy Centered on Orion® Orion Argus Research Execute Orion clinical and R&D programs Positive interim results of six subject U.S. Early Feasibility Study at UCLA and Baylor R&D to iterate cortical prosthesis and external components in preparation for pivotal trial Orion designated FDA Breakthrough Device for expedited pathway to commercialization Improve the artificial vision user experience Multiple research projects to enhance visual acuity and create a richer user experience Examples include object and facial recognition, distance filtering, thermal imaging and eye tracking Technologies can be integrated in both Argus and Orion platforms Leverage Argus II technology and core competencies Leverage key competencies in clinical, rehabilitation and market development to benefit Orion Restructuring implemented to reduce commercial and production activities We remain committed to supporting all Argus users
Orion® Extends our Leadership Position in Artificial Vision Established technology with 10+ years proven implant durability Proprietary algorithms for artificial vision Over 85 issued U.S. patents for Orion Orion leverages Argus II technology platform Argus is an established therapy in over a dozen markets globally Achieved highest CMS outpatient reimbursement rate in U.S. at $152.5k for 2019 CMS’ recent proposals to provide reimbursement for Breakthrough Devices on approval are encouraging Market access & reimbursement expertise Effective patient outreach programs and a multi-step patient qualification process applicable to Argus and Orion Large and growing U.S. database that includes over 1,000 potential Orion candidates Effective patient outreach & screening with U.S. patient database Centers of Excellence model to build regional artificial vision centers for Argus and Orion Best practices development and pioneered post-surgical rehabilitation to improve patient satisfaction and outcomes for all artificial vision patients Centers of Excellence (COE) model and artificial vision rehabilitation
Research Projects to Enhance User Experience Eye Tracking Move the implant field of view in conjunction with the movement of the user’s eyes Thermal Imaging Infrared imaging would allow users to visualize warm objects such as people in a room Depth-Based Decluttering Allow users to filter out objects further than a defined distance Research projects to drive potential benefit for Argus and Orion users Object & Facial Recognition Receive additional auditory and/or haptic information integrated with their artificial vision
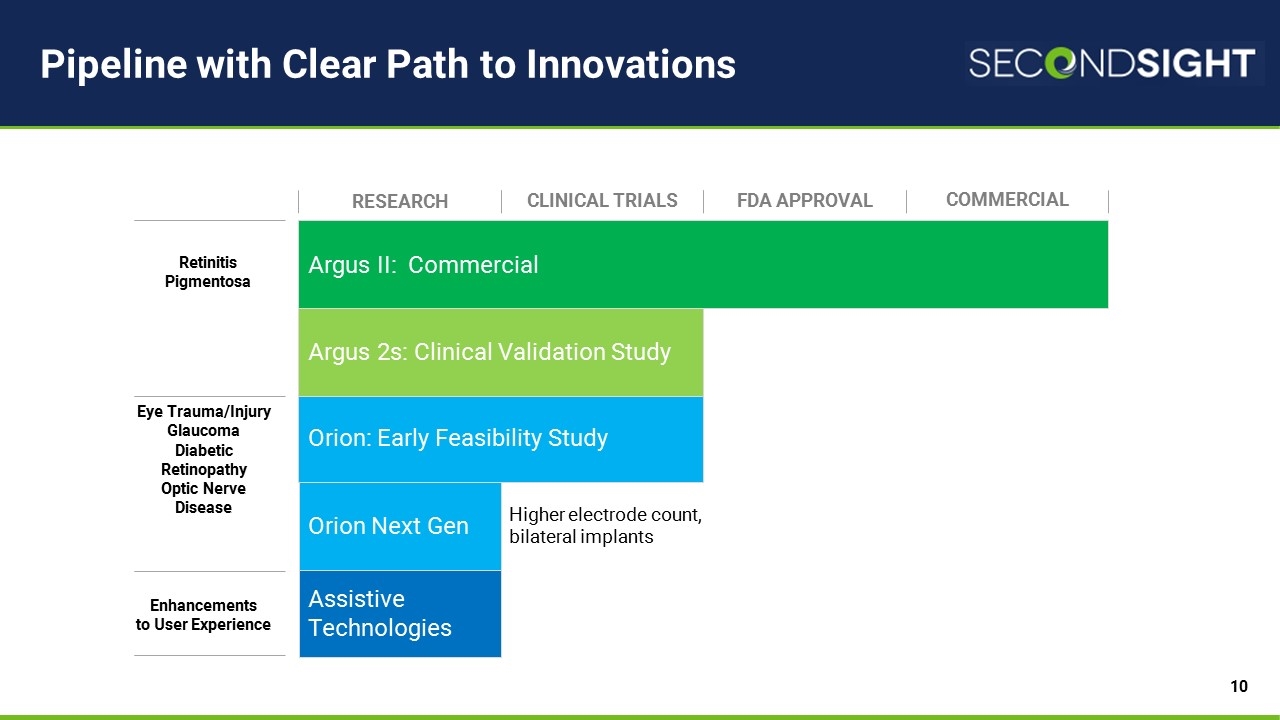
Pipeline with Clear Path to Innovations Argus II: Commercial Orion: Early Feasibility Study Argus 2s: Clinical Validation Study Research CLINICAL TRIALS FDA APPROVAL COMMERCIAL Assistive Technologies Retinitis Pigmentosa Eye Trauma/Injury Glaucoma Diabetic Retinopathy Optic Nerve Disease Enhancements to User Experience Orion Next Gen Higher electrode count, bilateral implants
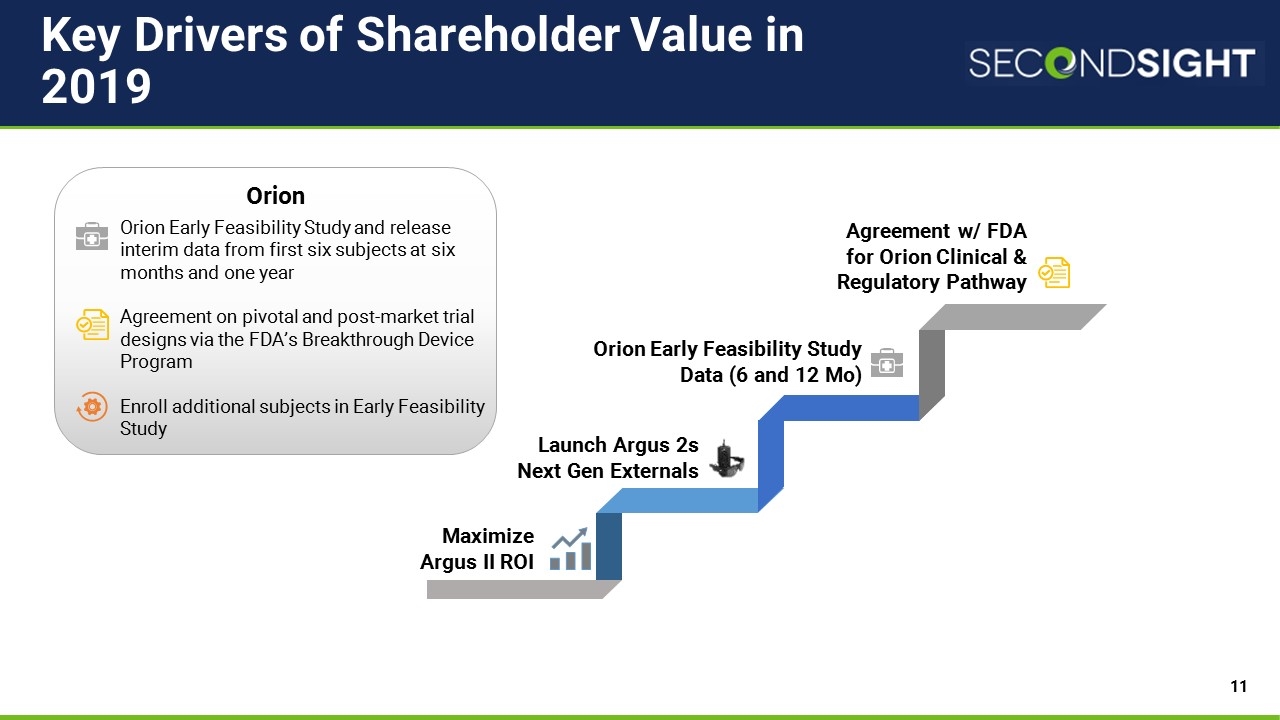
Key Drivers of Shareholder Value in 2019 Orion Agreement w/ FDA for Orion Clinical & Regulatory Pathway Maximize Argus II ROI Orion Early Feasibility Study Data (6 and 12 Mo) Orion Early Feasibility Study and release interim data from first six subjects at six months and one year Agreement on pivotal and post-market trial designs via the FDA’s Breakthrough Device Program Enroll additional subjects in Early Feasibility Study Launch Argus 2s Next Gen Externals
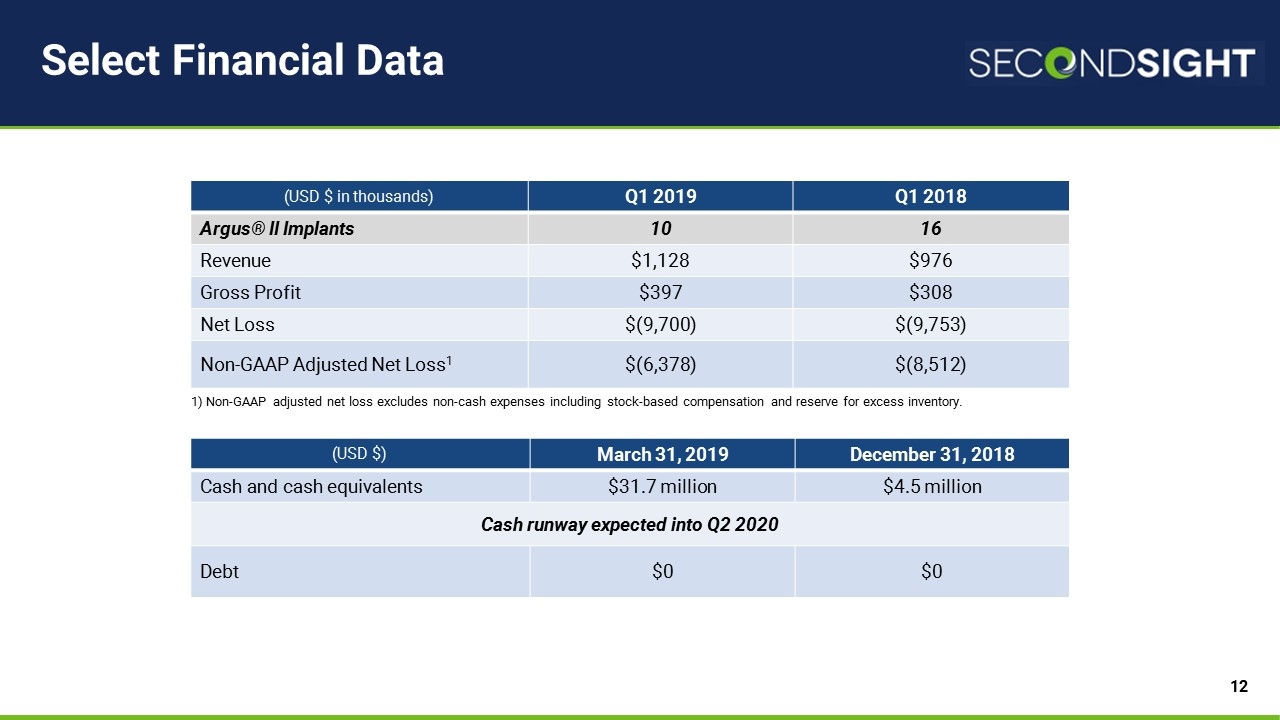
Select Financial Data 1) Non-GAAP adjusted net loss excludes non-cash expenses including stock-based compensation and reserve for excess inventory. (USD $ in thousands) Q1 2019 Q1 2018 Argus® II Implants 10 16 Revenue $1,128 $976 Gross Profit $397 $308 Net Loss $(9,700) $(9,753) Non-GAAP Adjusted Net Loss1 $(6,378) $(8,512) (USD $) March 31, 2019 December 31, 2018 Cash and cash equivalents $31.7 million $4.5 million Cash runway expected into Q2 2020 Debt $0 $0

Experienced Leadership Team Will McGuire President & CEO 20+ years experience in medical device industry Extensive leadership experience across both public and private companies Most recently as President of Americas Commercial for Volcano Corporation (acquired by Phillips for $1.2B in 2015). John Blake Chief Financial Officer 15+ years experience in public company finance and accounting Extensive transactional and M&A experience in high-growth medical device and biotech Former leadership roles at aTyr Pharma and Volcano Corporation (acquired by Phillips for $1.2B in 2015) Steve Okland Chief Commercial Officer 25+ years experience in medical device industry Commercialization leadership roles at Medivance (acquired by Bard Medical for $250M in 2011), Spectranetics, Boston Scientific Corporation and Johnson & Johnson Medical William Patrick Ryan Chief Operating Officer 20+ years experience in medical device industry Extensive COO leadership experience (Synaptive Medical, Lucerno Dynamics, Insulet Corporation, Alphatec Spine) Leadership positions at Guidant & Abbott Vascular

Second Sight Investment Highlights Orion is a large market opportunity that’s more than 50x the current RP market Potential to treat nearly all forms of blindness including diabetic retinopathy, glaucoma, optic nerve disease and eye injury Six subject feasibility study at UCLA and Baylor underway with good early results and additional interim data in 2019 FDA Breakthrough Device designation provides expedited regulatory and clinical pathway Orion leverages Argus® II technological backbone Orion leverages Argus technology including implantable array, externals and proprietary software / algorithms for creating artificial vision Orion also leverages reimbursement success, patient outreach and screening expertise as well as artificial vision rehabilitation competencies Future technologies under development beneficial to Argus II and Orion Eye-tracking, thermal imaging and depth-based decluttering provide improved or more useful vision Object recognition and facial recognition create enhanced user experience

Contacts Second Sight Medical Products, Inc. 12744 San Fernando Road Suite 400 Sylmar, CA 91342 Main: 818-833-5000 www.secondsight.com Will McGuire President & CEO Direct: 818-833-5040 wmcguire@secondsight.com Institutional Investor Relations Lisa Wilson President In-Site Communications, Inc. Direct: 212-452-2793 lwilson@insitecony.com Retail Investor Relations Greg Falesnik Managing Director MZ North America Direct: 949-385-6449 Greg.Falesnik@mzgroup.us