

Second Sight Medical Products, Inc. (NASDAQ: EYES) Discover Life in a New Light® 2019 Wells Fargo Healthcare Conference September 4, 2019 Ex 99.1

The following slides and any accompanying oral presentation contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are intended to be covered by the "safe harbor" created by those sections. All statements in this release that are not based on historical fact are "forward looking statements." These statements may be identified by words such as "estimates," "anticipates," "projects," "plans" or "planned," "strategy," “goal," "seeks," "may," "will," "expects," "intends," "believes," "should," and similar expressions, or the negative versions thereof, and which also may be identified by their context. All statements that address operating performance or events or developments that Second Sight expects or anticipates will occur in the future, such as stated objectives or goals, or that are not otherwise historical facts, are forward-looking statements. While management has based any forward-looking statements included in this release on its current expectations, the information on which such expectations were based may change. Forward-looking statements involve inherent risks and uncertainties which could cause actual results to differ materially from those in the forward-looking statements as a result of various factors, including those risks and uncertainties described in the Risk Factors and in Management's Discussion and Analysis of Financial Condition and Results of Operations sections of our Annual Report, on Form 10-K, filed on March 19, 2019, our most recent 10-Q, filed on August 6, 2019 and our other reports filed from time to time with the Securities and Exchange Commission. We urge you to consider those risks and uncertainties in evaluating our forward-looking statements. We caution readers not to place undue reliance upon any such forward-looking statements, which speak only as of the date made. Except as otherwise required by the federal securities laws, we disclaim any obligation or undertaking to publicly release any updates or revisions to any forward-looking statement contained herein (or elsewhere) to reflect any change in our expectations with regard thereto, or any change in events, conditions, or circumstances on which any such statement is based. Forward Looking Statements
Our Mission: To become the standard of care for profound blindness The Leader in Neuromodulation for Blindness The first of its kind We are developing the world’s first commercial Visual Cortical Prosthesis Currently in human trials Early feasibility study at Ronald Reagan UCLA Medical Center (UCLA) & Baylor College of Medicine (Baylor) Leveraging broad experience in artificial vision Argus II Retinal Prosthesis with over 300 users worldwide Broadest patent portfolio with 300+ patents issued or pending Unique artificial vision training competencies Proven market access capabilities
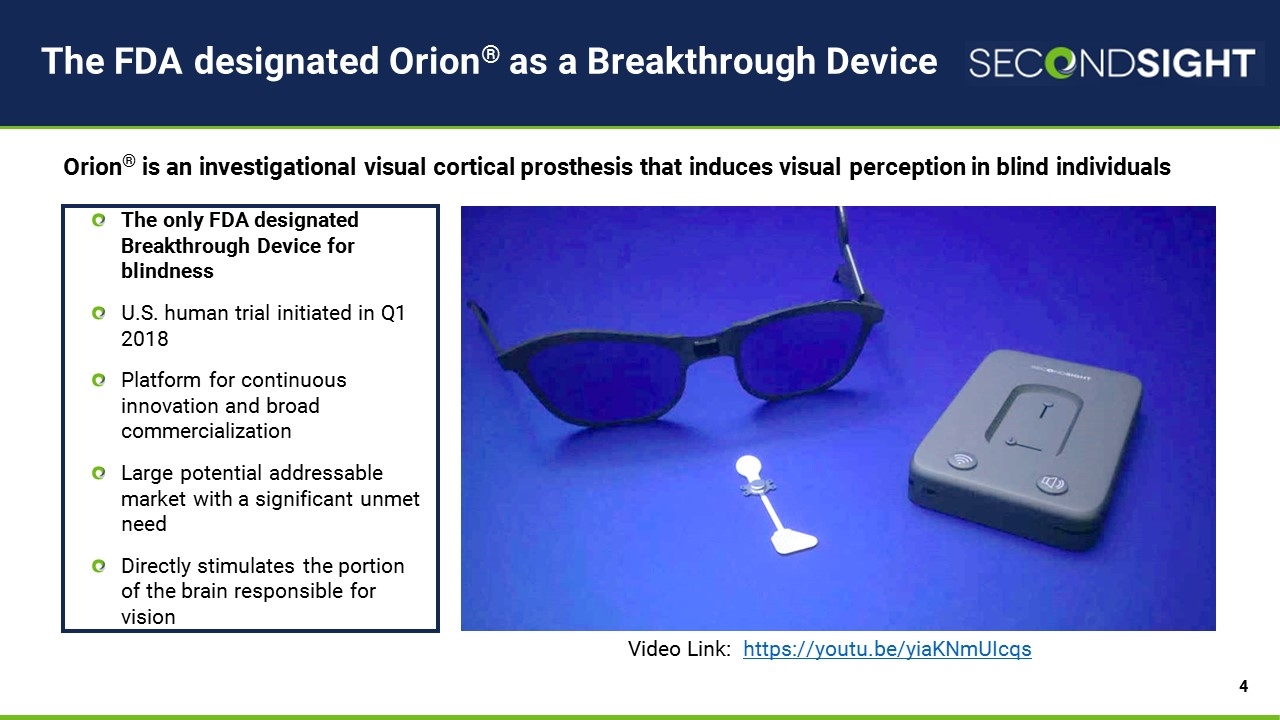
The FDA designated Orion® as a Breakthrough Device The only FDA designated Breakthrough Device for blindness U.S. human trial initiated in Q1 2018 Platform for continuous innovation and broad commercialization Large potential addressable market with a significant unmet need Directly stimulates the portion of the brain responsible for vision Orion® is an investigational visual cortical prosthesis that induces visual perception in blind individuals Video Link: https://youtu.be/yiaKNmUIcqs
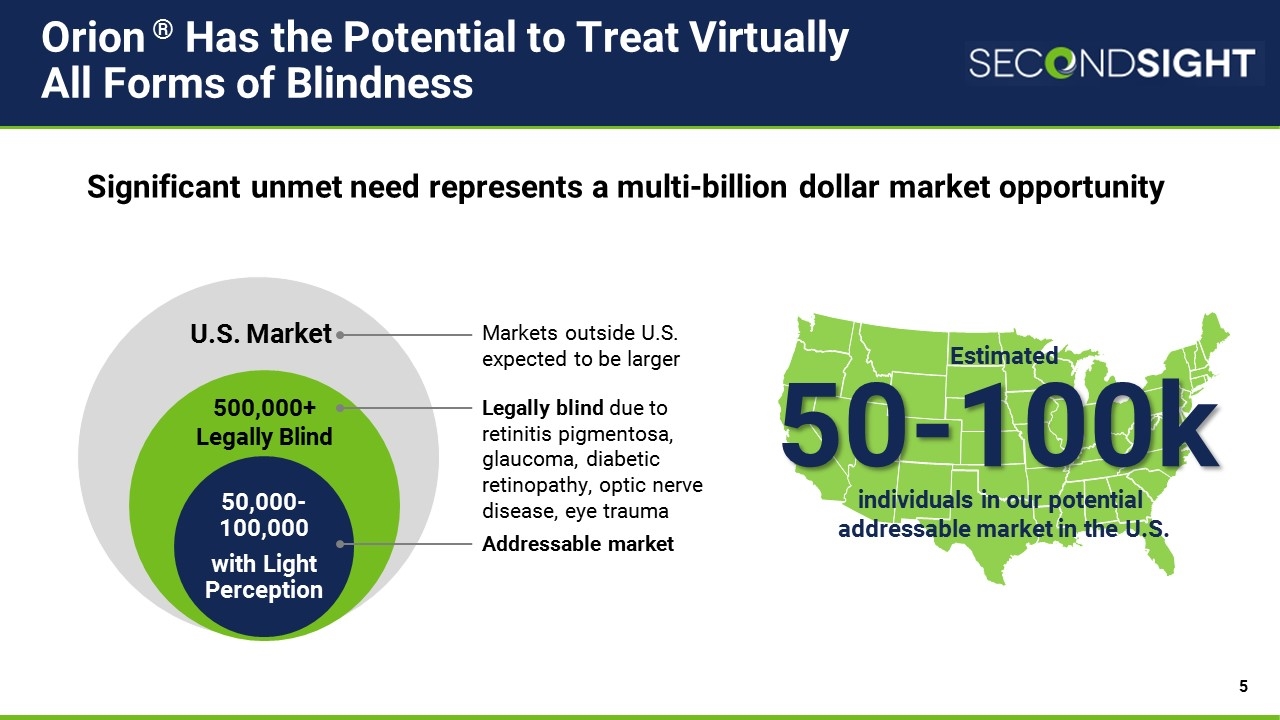
Estimated individuals in our potential addressable market in the U.S. Orion ® Has the Potential to Treat Virtually All Forms of Blindness U.S. Market 50,000-100,000 with Light Perception 500,000+ Legally Blind Addressable market Legally blind due to retinitis pigmentosa, glaucoma, diabetic retinopathy, optic nerve disease, eye trauma Significant unmet need represents a multi-billion dollar market opportunity 50-100k Markets outside U.S. expected to be larger

Growth Strategy Centered on Orion® Orion Core Competencies Research Execute Orion clinical and R&D programs Positive six- and 12-month results of six subject U.S. Early Feasibility Study at UCLA and Baylor R&D iterating cortical prosthesis and external components in preparation for pivotal clinical trial and future commercialization Orion designated FDA Breakthrough Device for expedited pathway to commercialization Innovate and improve the artificial vision user experience Multiple research projects to enhance visual acuity and create a richer user experience Examples distance filtering, thermal imaging, eye tracking and facial & object recognition Technologies can be integrated in both Argus and Orion platforms Focusing resources on Orion and leveraging key market development competencies Production of Argus II to cease at end of 2019; we remain committed to supporting Argus implanting centers and users Production resources preparing for high-volume manufacturing of Orion Demonstrated competencies in market access/reimbursement and patient recruitment
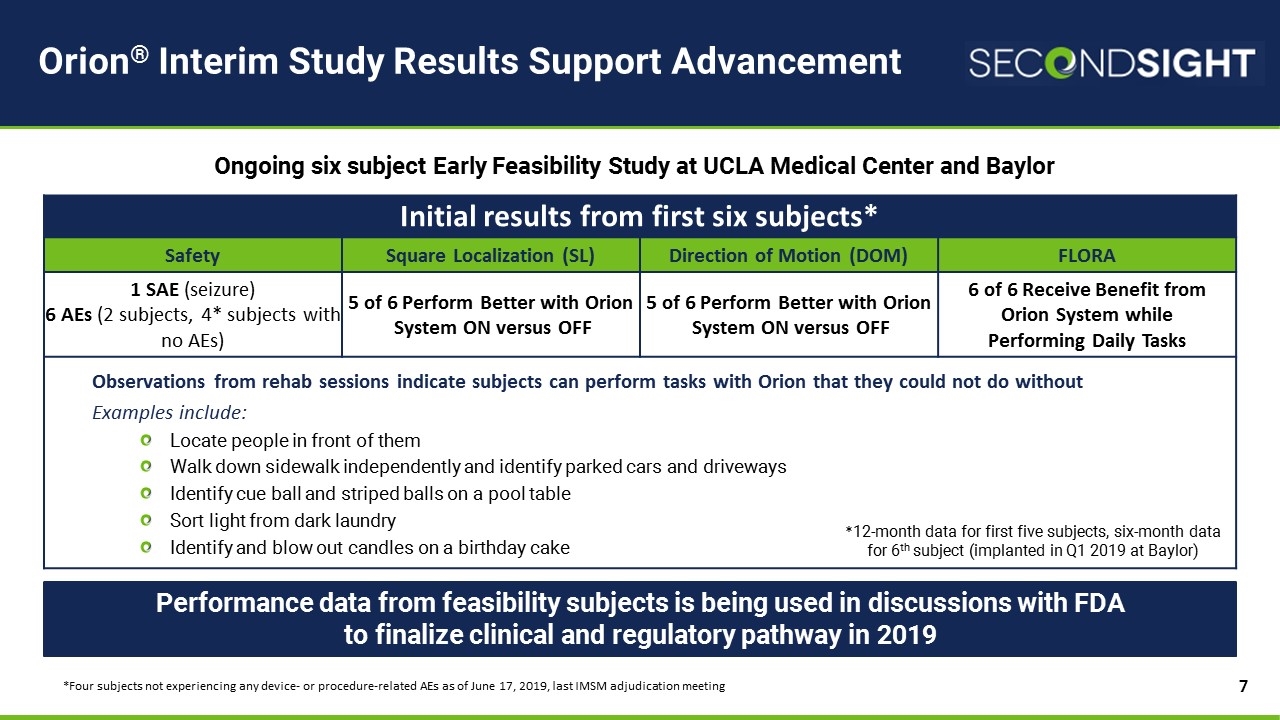
Orion® Interim Study Results Support Advancement Ongoing six subject Early Feasibility Study at UCLA Medical Center and Baylor Performance data from feasibility subjects is being used in discussions with FDA to finalize clinical and regulatory pathway in 2019 Initial results from first six subjects* Safety Square Localization (SL) Direction of Motion (DOM) FLORA 1 SAE (seizure) 6 AEs (2 subjects, 4* subjects with no AEs) 5 of 6 Perform Better with Orion System ON versus OFF 5 of 6 Perform Better with Orion System ON versus OFF 6 of 6 Receive Benefit from Orion System while Performing Daily Tasks Observations from rehab sessions indicate subjects can perform tasks with Orion that they could not do without Examples include: Locate people in front of them Walk down sidewalk independently and identify parked cars and driveways Identify cue ball and striped balls on a pool table Sort light from dark laundry Identify and blow out candles on a birthday cake *12-month data for first five subjects, six-month data for 6th subject (implanted in Q1 2019 at Baylor) *Four subjects not experiencing any device- or procedure-related AEs as of June 17, 2019, last IMSM adjudication meeting

R&D On-track for Commercial Version Changes to the Orion implant are focused on the electronics case to reduce the thickness and improve impact resistance No planned changes to the electrode array design or the materials Improvements to the Orion externals focus on a faster processor and more memory Other changes are for improved water and impact resistance, and improved ergonomics None of the proposed changes to the implant or the externals impact functionality
Research Projects to Enhance User Experience Eye Tracking Move the implant field of view in conjunction with the movement of the user’s eyes Thermal Imaging Infrared imaging would allow users to visualize warm objects such as people in a room Depth-Based Decluttering Allow users to filter out objects further than a defined distance Research projects to drive potential benefit for Orion users Object & Facial Recognition Receive additional auditory and/or haptic information integrated with their artificial vision
Orion® Extends our Leadership Position in Artificial Vision Established technology with 10+ years proven implant durability Proprietary algorithms for artificial vision Over 85 issued U.S. patents for Orion Orion leverages Argus II technology platform Argus II is an established therapy in over a dozen markets globally Achieved highest CMS outpatient reimbursement rate for a device and related procedure in U.S. at $152,500 for 2019 CMS’ efforts to provide reimbursement for Breakthrough Devices on approval apply to Orion Leaders in market access and reimbursement Effective patient outreach programs with a multi-step patient qualification process Large and growing U.S. database that includes over 1,000 potential Orion candidates Effective patient outreach and screening with large U.S. patient database Partnered with 20 premier hospitals in the U.S. to build regional artificial vision centers Developed best practices for post-surgical training and rehabilitation to improve patient satisfaction and outcomes Established commercial channel with a Centers of Excellence model
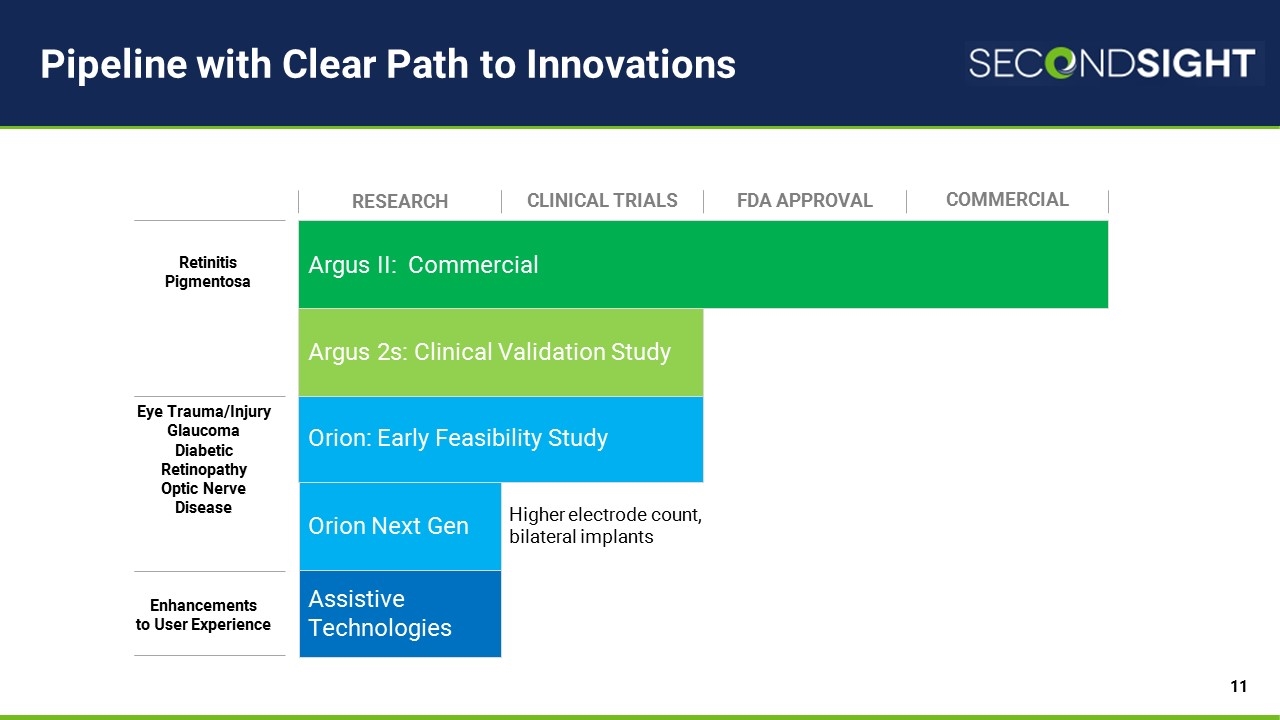
Pipeline with Clear Path to Innovations Argus II: Commercial Orion: Early Feasibility Study Argus 2s: Clinical Validation Study Research CLINICAL TRIALS FDA APPROVAL COMMERCIAL Assistive Technologies Retinitis Pigmentosa Eye Trauma/Injury Glaucoma Diabetic Retinopathy Optic Nerve Disease Enhancements to User Experience Orion Next Gen Higher electrode count, bilateral implants

Experienced Leadership Team Will McGuire President & CEO 20+ years experience in medical device industry Extensive leadership experience across both public and private companies Most recently as President of Americas Commercial for Volcano Corporation (acquired by Phillips for $1.2B in 2015). John Blake Chief Financial Officer 15+ years experience in public company finance and accounting Over $1.6 billion in transactional experience including IPOs and M&A in high-growth medical device and biotech Former senior leadership roles at aTyr Pharma and Volcano Corporation (acquired by Phillips for $1.2B in 2015) Steve Okland Chief Commercial Officer 25+ years experience in medical device industry Commercialization leadership roles at Medivance (acquired by Bard Medical for $250M in 2011), Spectranetics, Boston Scientific Corporation and Johnson & Johnson Medical William Patrick Ryan Chief Operating Officer 20+ years experience in medical device industry Extensive COO leadership experience (Synaptive Medical, Lucerno Dynamics, Insulet Corporation, Alphatec Spine) Leadership positions at Guidant & Abbott Vascular Jessy Dorn Vice President Clinical and Scientific Research 13+ years experience in artificial vision, neurostimulation and psychophysics Principal Investigator (PI) for NIH BRAIN Initiative grant funding ORION PI/Study Director for multiple Argus II clinical trials and nonsignificant risk studies Co-author of over 20 Argus II and Orion peer-reviewed publications Inventor on 6 patents related to Argus II and Orion Amit Kukreja Vice President Global Reimbursement and Market Access 14+ years experience in medical devices industry and healthcare strategy consulting Extensive global experience in market access, pricing & payor marketing strategy for disruptive medical devices Strategic operational roles at Synergus Consulting, Simon Kucher & Partners and Godrej & Boyce
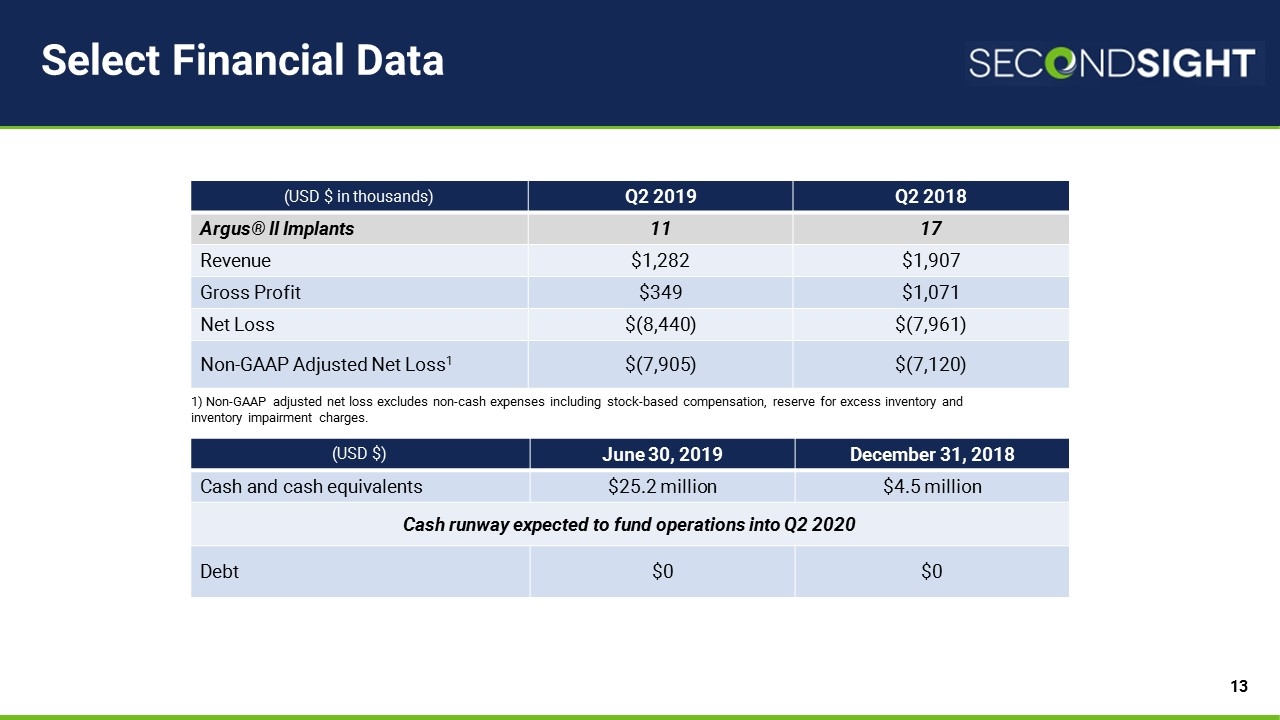
Select Financial Data 1) Non-GAAP adjusted net loss excludes non-cash expenses including stock-based compensation, reserve for excess inventory and inventory impairment charges. (USD $ in thousands) Q2 2019 Q2 2018 Argus® II Implants 11 17 Revenue $1,282 $1,907 Gross Profit $349 $1,071 Net Loss $(8,440) $(7,961) Non-GAAP Adjusted Net Loss1 $(7,905) $(7,120) (USD $) June 30, 2019 December 31, 2018 Cash and cash equivalents $25.2 million $4.5 million Cash runway expected to fund operations into Q2 2020 Debt $0 $0

Execution on Track in 2019 Second Half 2019 Objectives Execute R&D implant and externals projects for commercial version of Orion Finalize agreement with the FDA regarding Orion’s clinical and regulatory path Engage and expand discussions with CMS and private payors while developing a comprehensive reimbursement strategy for Orion in the U.S. Submit Argus 2s, our next-gen externals, to the FDA for U.S. regulatory approval Add key talent to support our Orion programs and develop a plan for high-volume manufacturing

Second Sight Investment Highlights Orion potentially addresses a significant unmet need for 50,000 to 100,000 individuals in the U.S. Potential to treat nearly all forms of blindness including retinitis pigmentosa, diabetic retinopathy, glaucoma, optic nerve disease and eye injury Six subject feasibility study at UCLA and Baylor has positive results that support advancement Orion leverages Argus® II technological backbone and is designated a Breakthrough Device by FDA Orion leverages Argus technology including implantable array, externals and proprietary software / algorithms for creating artificial vision FDA Breakthrough Device designation provides expedited regulatory and clinical pathway Future technologies under development enhance or improve artificial vision experience Different array designs including a higher electrode count array and the associated electronics Eye-tracking, thermal imaging, depth-based decluttering and object and/or recognition facial recognition provide improved or more useful vision Large U.S. patient database and proven reimbursement and market access capabilities Demonstrated patient outreach and screening expertise with over 1,000 potential Orion patients in a growing U.S. database Orion will benefit from company’s efforts to secure an Argus II reimbursement rate of $152,500 in U.S. as well as CMS initiatives to establish reimbursement for FDA Breakthrough Devices upon regulatory approval.

Contacts Second Sight Medical Products, Inc. 12744 San Fernando Road Suite 400 Sylmar, CA 91342 Main: 818-833-5000 www.secondsight.com Will McGuire President & CEO Direct: 818-833-5040 wmcguire@secondsight.com Institutional Investor Relations Lisa Wilson President In-Site Communications, Inc. Direct: 212-452-2793 lwilson@insitecony.com Retail Investor Relations Greg Falesnik Managing Director MZ North America Direct: 949-385-6449 Greg.Falesnik@mzgroup.us