

Second Sight Medical Products, Inc. (NASDAQ: EYES) Discover Life in a New Light® Investor Presentation Filed pursuant to Rule 433 Dated April 29 , 2020 Issuer Free Writing Prospectus supplementing the Preliminary Prospectus Supplement dated April 29 , 2020 and the Prospectus dated November 9, 2017 Registration No. 333‐221228

• The following slides and any accompanying oral presentation contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are intended to be covered by the "safe harbor" created by those sections. All statements in this release that are not based on historical fact are "forward looking statements." These statements may be identified by words such as "estimates," "anticipates," "projects," "plans" or "planned," "strategy," “goal," "seeks," "may," "will," "expects," "intends," "believes," "should," and similar expressions, or the negative versions thereof, and which also may be identified by their context. All statements that address operating performance or events or developments that Second Sight expects or anticipates will occur in the future, such as stated objectives or goals, or that are not otherwise historical facts, are forward-looking statements. While management has based any forward-looking statements included in this release on its current expectations, the information on which such expectations were based may change. Forward-looking statements involve inherent risks and uncertainties which could cause actual results to differ materially from those in the forwardlooking statements as a result of various factors, including those risks and uncertainties described in the Risk Factors and in Management's Discussion and Analysis of Financial Condition and Results of Operations sections of our Annual Report, on Form 10-K, filed on March 19, 2020 and our other reports filed from time to time with the Securities and Exchange Commission. We urge you to consider those risks and uncertainties in evaluating our forward-looking statements. We caution readers not to place undue reliance upon any such forward-looking statements, which speak only as of the date made. Except as otherwise required by the federal securities laws, we disclaim any obligation or undertaking to publicly release any updates or revisions to any forward-looking statement contained herein (or elsewhere) to reflect any change in our expectations with regard thereto, or any change in events, conditions, or circumstances on which any such statement is based. • Second Sight Medical Products, Inc. has filed a registration statement (including the prospectus supplement and the accompanying prospectus) with the Securities and Exchange Commission (the “SEC”) with respect to the offering of shares of the Company’s common stock to which this communication relates. Before you invest, you should read the prospectus supplement and the accompanying prospectus in the registration statement (Registration No. 333-221228) and the other documents Second Sight Medical Products, Inc. has filed with the SEC and incorporated by reference for more complete information about Second Sight Medical Products, Inc. and this offering. You may get these documents for free by visiting EDGAR on the SEC website at http://sec.gov. Copies of the prospectus supplement and accompanying prospectus may also be obtained from ThinkEquity, a division of Fordham Financial Management, Inc., Prospectus Department, 17 State Street, 22nd Floor New York NY 10004, telephone (877) 436-3673, email: prospectus@think-equity.com • This communication should be read in conjunction with the Preliminary Prospectus Supplement and the accompanying prospectus. The information in this communication supersedes the information in the Preliminary Prospectus Supplement and the accompanying prospectus to the extent inconsistent with the information in the Preliminary Prospectus Supplement and the accompanying prospectus. Forward Looking Statements Free Writing Prospectus
Highlights 3 Established technology with 10+ years proven implant durability Proprietary algorithms for artificial vision Over 85 issued U.S. patents for Orion, over 300 patents in total with 40 pending applications Orion leverages Argus II technology platform Argus II is an established therapy in over a dozen markets globally; over 300 implants to date Achieved highest CMS outpatient reimbursement rate for a device and related procedure in U.S. at $152,500 for 2019 CMS’ efforts to provide reimbursement for Breakthrough Devices on approval apply to Orion Leaders in market access and reimbursement 1st FDA approved retinal prosthesis Partnered with 20 premier hospitals in the U.S. to build regional artificial vision centers – Centers of Excellence Model Effective patient outreach and screening competencies – over 1,000 potential patients in current database Second Sight is the Global Leader in Artificial Vision
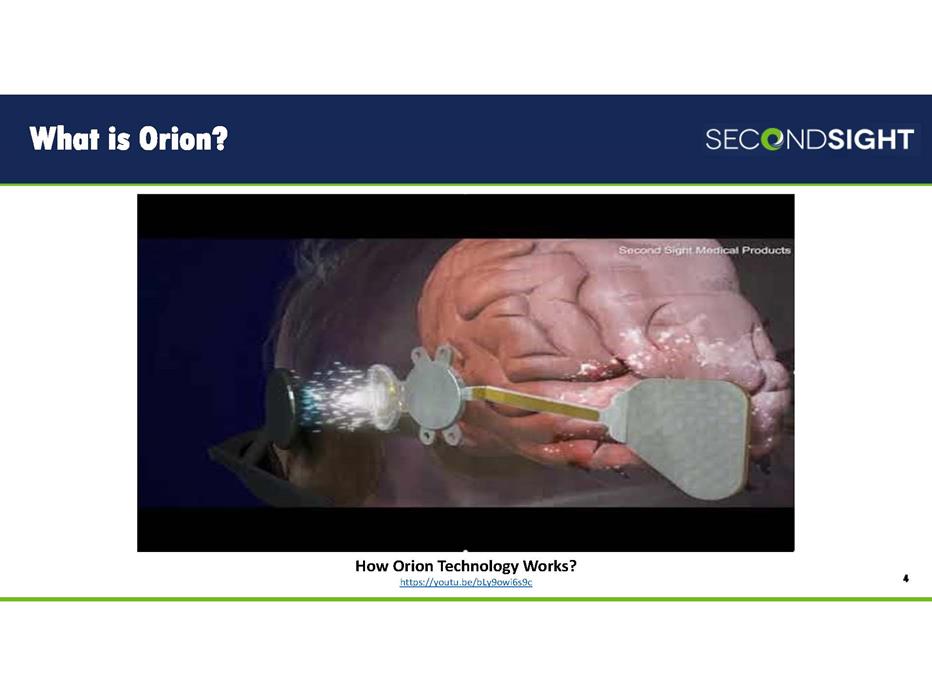
What is Orion? 4 How Orion Technology Works? https://youtu.be/bLy9owi6s9c

Orion Patient Stories 5 ABC 6 Philadelphia https://6abc.com/health/brain-implant-gives-blind-new-way-to-see-worldaround- them/5553255/ The Story of Medtech https://thestoryofmedtech.org/story/seeinganew- artificial-vision-richards-story CNET What the Future: Artificial Vision for the Blind https://www.cnet.com/videos/this‐machine‐creates‐artificial‐visionfor‐ the‐blind/
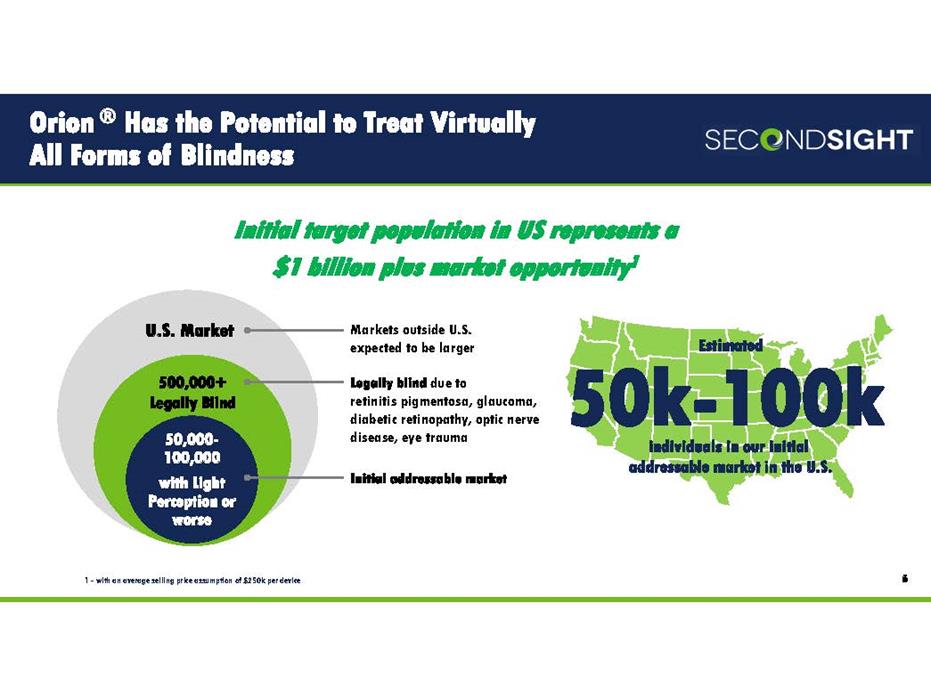
Estimated individuals in our initial addressable market in the U.S. Orion ® Has the Potential to Treat Virtually All Forms of Blindness 6 U.S. Market 50,000- 100,000 with Light Perception or worse 500,000+ Legally Blind Initial addressable market Legally blind due to retinitis pigmentosa, glaucoma, diabetic retinopathy, optic nerve disease, eye trauma Initial target population in US represents a $1 billion plus market opportunity1 50k-100k Markets outside U.S. expected to be larger 1 - with an average selling price assumption of $250k per device
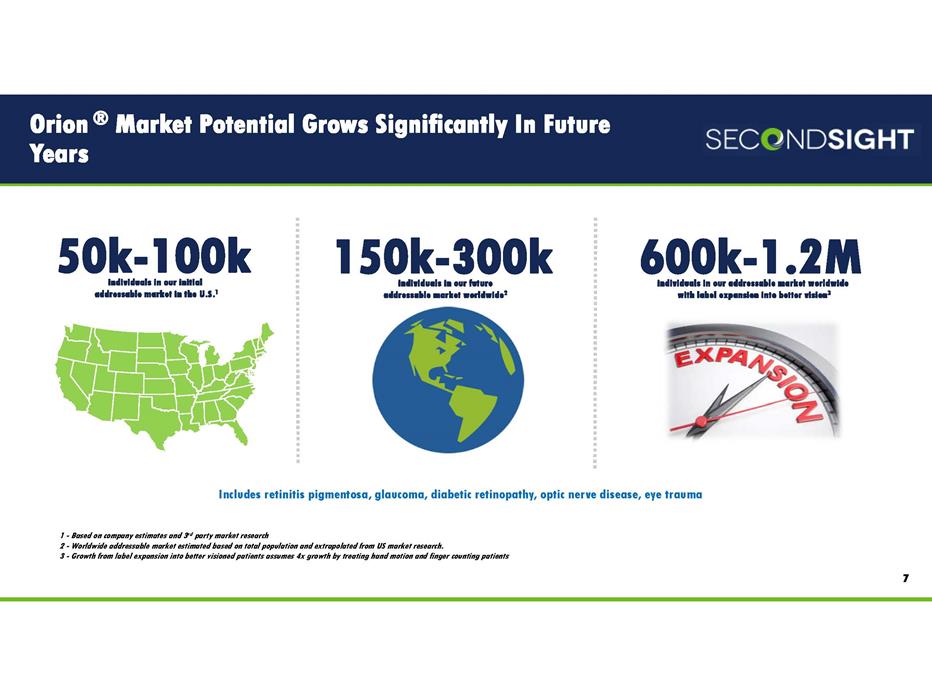
Orion ® Market Potential Grows Significantly In Future Years 7 individuals in our initial addressable market in the U.S.1 50k-100k individuals in our future addressable market worldwide2 150k-300k individuals in our addressable market worldwide with label expansion into better vision3 600k-1.2M 1 - Based on company estimates and 3rd party market research 2 - Worldwide addressable market estimated based on total population and extrapolated from US market research. 3 - Growth from label expansion into better visioned patients assumes 4x growth by treating hand motion and finger counting patients Includes retinitis pigmentosa, glaucoma, diabetic retinopathy, optic nerve disease, eye trauma
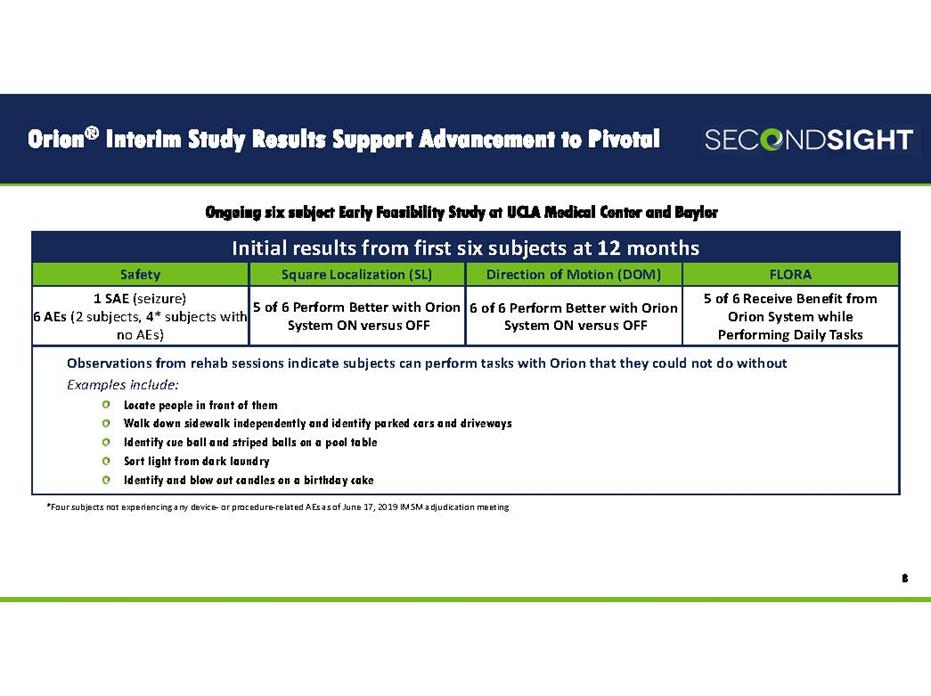
Orion® Interim Study Results Support Advancement to Pivotal 8 Ongoing six subject Early Feasibility Study at UCLA Medical Center and Baylor Initial results from first six subjects at 12 months Safety Square Localization (SL) Direction of Motion (DOM) FLORA 1 SAE (seizure) 6 AEs (2 subjects, 4* subjects with no AEs) 5 of 6 Perform Better with Orion System ON versus OFF 6 of 6 Perform Better with Orion System ON versus OFF 5 of 6 Receive Benefit from Orion System while Performing Daily Tasks Observations from rehab sessions indicate subjects can perform tasks with Orion that they could not do without Examples include: Locate people in front of them Walk down sidewalk independently and identify parked cars and driveways Identify cue ball and striped balls on a pool table Sort light from dark laundry Identify and blow out candles on a birthday cake *Four subjects not experiencing any device‐ or procedure‐related AEs as of June 17, 2019 IMSM adjudication meeting
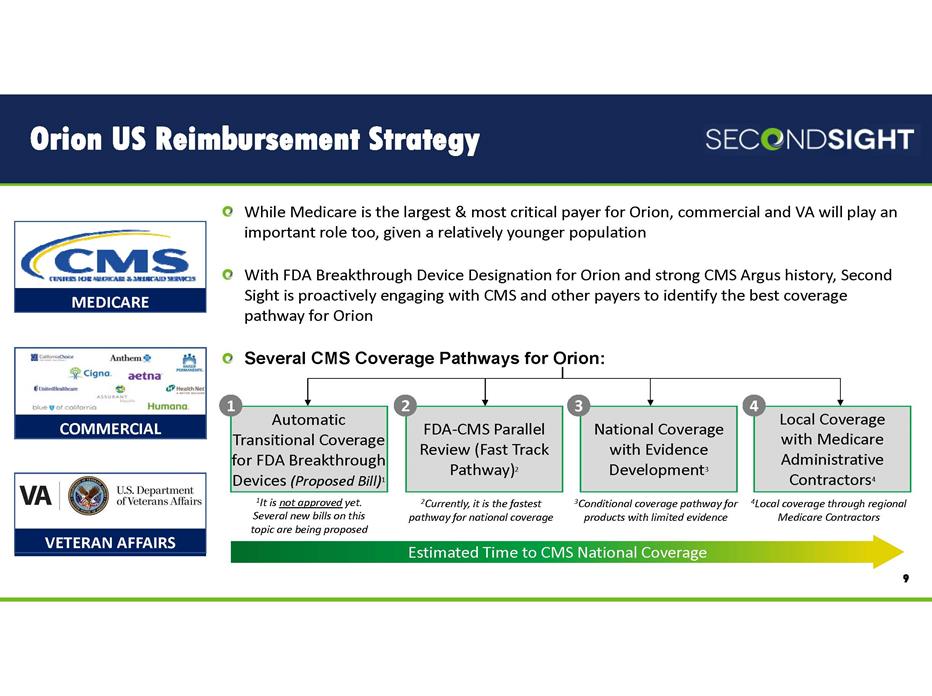
Orion US Reimbursement Strategy 9 While Medicare is the largest & most critical payer for Orion, commercial and VA will play an important role too, given a relatively younger population With FDA Breakthrough Device Designation for Orion and strong CMS Argus history, Second Sight is proactively engaging with CMS and other payers to identify the best coverage pathway for Orion Several CMS Coverage Pathways for Orion: MEDICARE COMMERCIAL VETERAN AFFAIRS FDA‐CMS Parallel Review (Fast Track Pathway)2 National Coverage with Evidence Development3 Local Coverage with Medicare Administrative Contractors4 Estimated Time to CMS National Coverage 1It is not approved yet. Several new bills on this topic are being proposed 2Currently, it is the fastest pathway for national coverage 3Conditional coverage pathway for products with limited evidence 4Local coverage through regional Medicare Contractors 1 2 3 4 Automatic Transitional Coverage for FDA Breakthrough Devices (Proposed Bill)1

KEY MILESTONES TO COMMERCIALIZATION • FLORA‐20 validation • FDA agreement on pivotal trial design • Submit IDE for Orion II • IDE approval • 1st Orion II implant in pivotal trial • Last Orion II implant in pivotal trial • Study follow‐up period (12 months postimplant) • Interim study results available • PMA submission • PMA approval • Commercialization Orion Regulatory Pathway – PMA Confidential 10
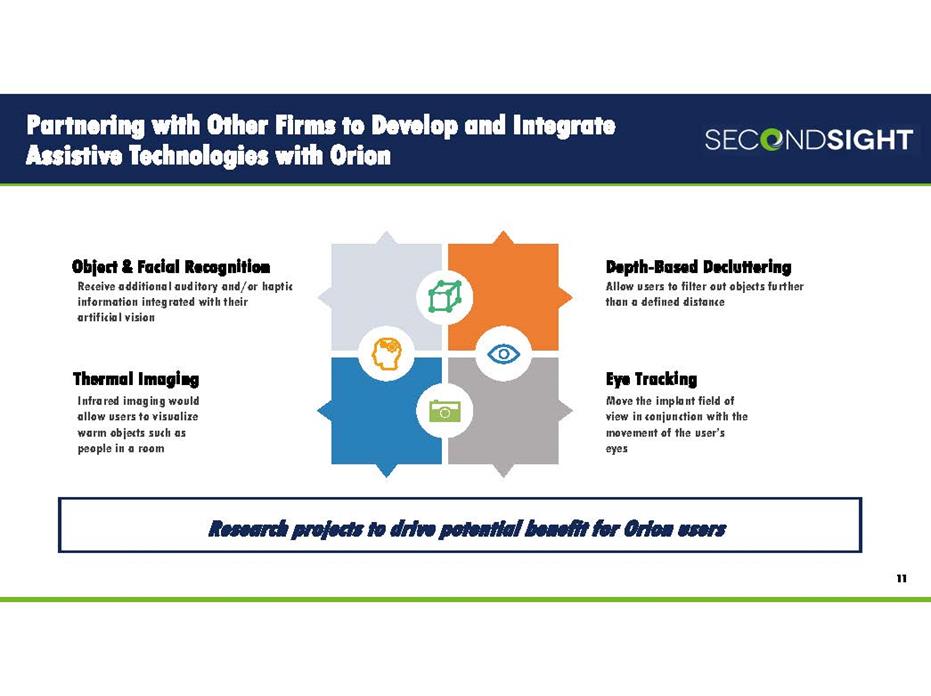
Partnering with Other Firms to Develop and Integrate Assistive Technologies with Orion Eye Tracking Move the implant field of view in conjunction with the movement of the user’s eyes Thermal Imaging Infrared imaging would allow users to visualize warm objects such as people in a room Depth-Based Decluttering Allow users to filter out objects further than a defined distance Research projects to drive potential benefit for Orion users 11 Object & Facial Recognition Receive additional auditory and/or haptic information integrated with their artificial vision
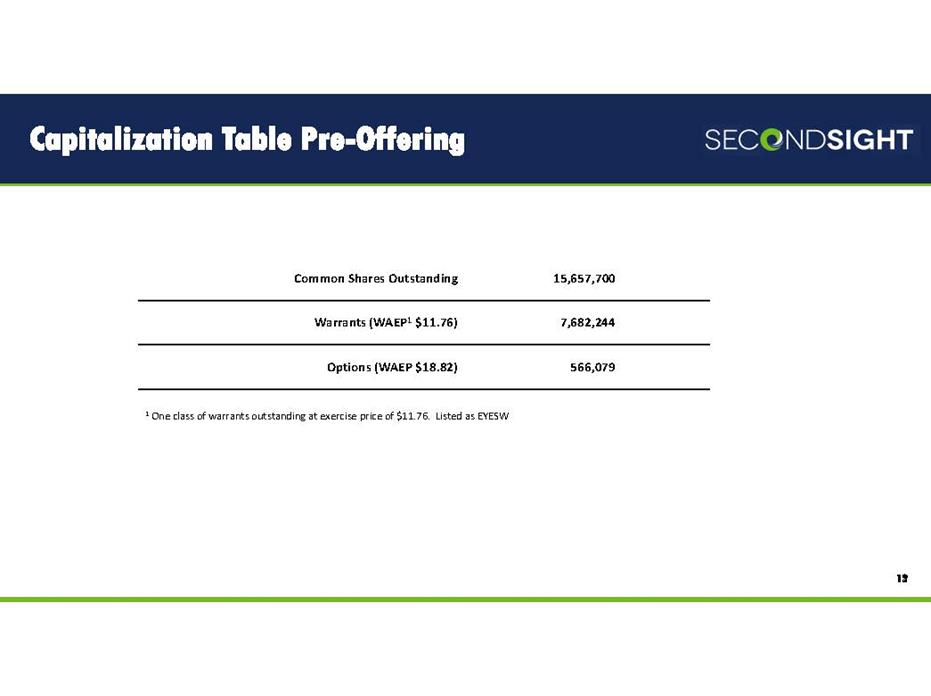
Capitalization Table Pre-Offering 12 Common Shares Outstanding 15,657,700 Warrants (WAEP1 $11.76) 7,682,244 Options (WAEP $18.82) 566,079 1 One class of warrants outstanding at exercise price of $11.76. Listed as EYESW

Second Sight Investment Highlights Orion represents a multi-billion dollar opportunity Potential to treat nearly all forms of blindness including retinitis pigmentosa, diabetic retinopathy, glaucoma, optic nerve disease and eye injury Six subject feasibility study at UCLA and Baylor has positive results that support advancement Orion leverages Argus® II technological backbone and is designated a Breakthrough Device by FDA Orion leverages Argus technology including implantable array, externals and proprietary software / algorithms for creating artificial vision FDA Breakthrough Device designation provides expedited regulatory and clinical pathway Future technologies enhance or improve artificial vision experience Different array designs including a higher electrode count array and the associated electronics Eye-tracking, thermal imaging, depth-based decluttering and object and/or recognition facial recognition provide improved or more useful vision Large U.S. patient database and proven reimbursement and market access capabilities Demonstrated patient outreach and screening expertise with over 1,000 potential Orion patients in a growing U.S. database Orion will benefit from company’s efforts to secure an Argus II reimbursement rate of $152,500 in U.S. as well as CMS initiatives to establish reimbursement for FDA Breakthrough Devices upon regulatory approval. 13

Contacts Second Sight Medical Products, Inc. 12744 San Fernando Road Suite 400 Sylmar, CA 91342 Main: 818-833-5000 www.secondsight.com John Blake CFO jblake@secondsight.com Institutional Investor Relations Lisa Wilson President In-Site Communications, Inc. Direct: 212-452-2793 lwilson@insitecony.com Retail Investor Relations Greg Falesnik Managing Director MZ North America Direct: 949-385-6449 Greg.Falesnik@mzgroup.us