
Exhibit 99.1

Nasdaq : EYES Investor Presentation NASDAQ: EYES January 2015

Nasdaq : EYES Forward Looking Statements This presentation contains certain forward - looking information about Second Sight that is intended to be covered by the safe harbor for "forward - looking statements" provided by the Private Securities Litigation Reform Act of 1995 , as amended . Forward - looking statements are statements that are not historical facts . Words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “strong,” “up coming,” and similar expressions are intended to identify forward - looking statements . These statements include, but are not limited to, statements regarding our ability to successfully develop and commercialize our products ; our ability to expand our long - term business opportunities ; financial projections and estimates and their underlying assumptions ; and future performance . In this document, we refer to information regarding potential markets for products and other industry data . We believe that all such information has been obtained from reliable sources that are customarily relied upon by companies in our industry . However, we have not independently verified any such information . Forward - looking statements may address the following subjects among others : expected products, applications, customers, technologies and performance, coverage and insurance reimbursements, results of clinical studies, success of research and development and our expectations concerning our business strategy . Forward - looking statements involve inherent risks and uncertainties which could cause actual results to differ materially from those in the forward - looking statements, as a result of various factors including those risks and uncertainties referred to in the risk factors section of the final prospectus filed with the Securities and Exchange Commission relating to our Registration Statement on Form S - 1 for our IPO . The audience is cautioned not to place undue reliance on these forward - looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward - looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non - occurrence of any events . 2
Nasdaq : EYES Second Sight ’ s Technology Platform 3 Second Sight’s Purpose : Restoring vision to the blind CNN Video: 'Bionic eye' lets blind man 'see' again http:// edition.cnn.com /video/data/2.0/video/international/2014/06/18/ spc - vital - signs - roger - pontz - bionic - eye.cnn.html

Nasdaq : EYES Second Sight ’ s Technology Platform 4 Second Sight’s Purpose : Restoring vision to the blind YouTube Video: First Implant at Duke Eye Center https://www.youtube.com/watch?v=CiyGOUHD2nI

Nasdaq : EYES 5 Corporate Summary • Restoring vision to the blind with the Argus II platform technology • Targeting significant addressable market of over 8 million people • Commercial stage – FDA, Canada, EU, & Turkey r egulatory approvals • Strong financial position – • Successful IPO in November 2014 raised $36.2M total gross. • Company is debt free • Shares outstanding – • Total shares outstanding : 35.3M • Fully diluted shares including options and warrants : 39.6M

Nasdaq : EYES Significant Addressable Markets 6 8 million patients globally are legally blind due to unpreventable causes 39 million people worldwide are legally blind
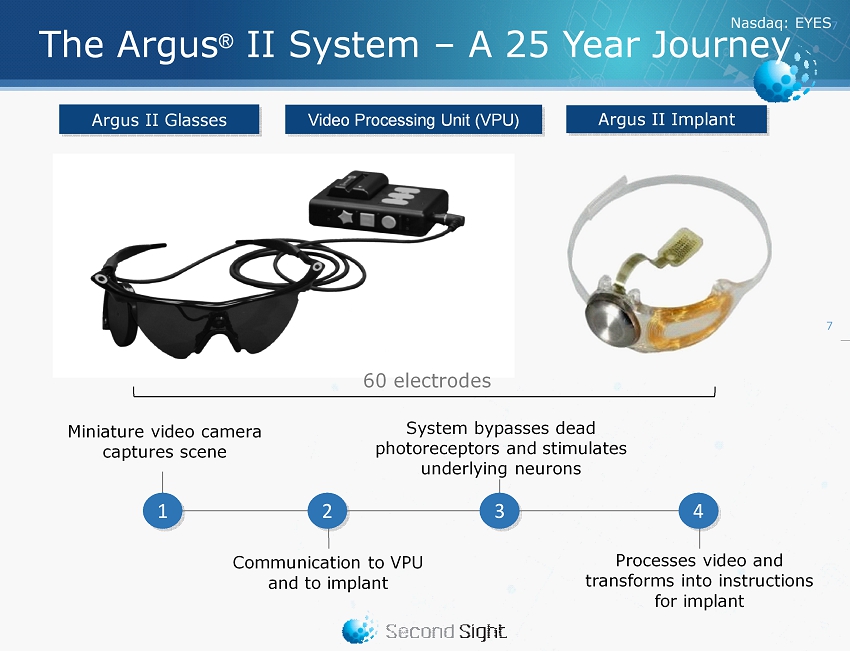
Nasdaq : EYES 7 Argus II Implant Argus II Glasses Video Processing Unit (VPU) The Argus ® II System – A 25 Year Journey System bypasses dead photoreceptors and stimulates underlying neurons Processes video and transforms into instructions for implant Miniature video camera captures scene Communication to VPU and to implant 1 3 4 2 60 electrodes 7

Nasdaq : EYES Experienced Management and Board Robert Greenberg Alfred E. Mann President, Chief Executive Officer & Director Chairman of the Board of Directors & Founder • Co - managed the Alfred E. Mann Foundation • Served as lead reviewer for IDEs and 510(k)s at the Office of Device Evaluation at FDA • Directs MannKind Corporation • Served as CEO of MiniMed (acquired by Medtronic) and Pacesetter Systems; Co - CEO of Advanced Bionics Corporation 8 William J. Link Aaron Mendelsohn Gregg Williams Director Director & Founder Director • Co - founder and managing director of Versant Ventures • Founded and served as Chairman and CEO of Chiron Vision • Experienced ophthalmology investor • Served on the board of Advanced Bionics • Founder and director of MRG, sold to Medtronic • CEO of Williams International Corporation • Director of General Aviation Manufacturers Association Decades of Experience Creating Value from Disruptive Technology
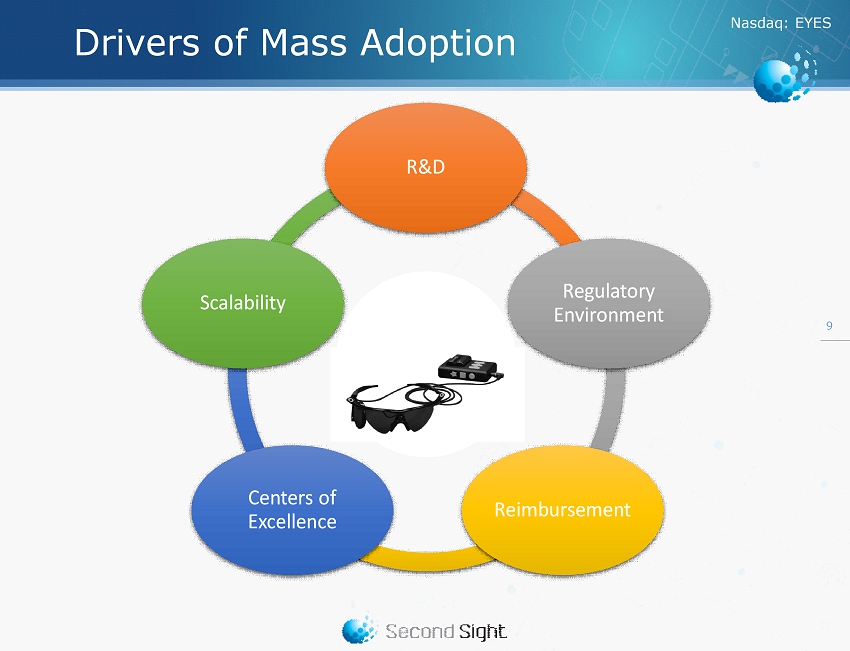
Nasdaq : EYES Drivers of Mass Adoption 9 R&D Regulatory Environment Reimbursement Centers of Excellence Scalability

Nasdaq : EYES 10 AMD Cortical prosthesis Software upgrade 2014 2015 2016 2017 2013 AMD First enrollment in feasibility study AMD Complete enrollment in feasibility AMD First enrollment in pivotal study AMD Complete enrollment in pivotal study Cortical First mechanical model animal implants Cortical First complete active device animal implants Cortical First enrollment in human feasibility trial Cortical Complete enrollment in human feasibility trial IPO Original Argus II FDA Approval Software Improvements Approved R&D and Regulatory Milestones Current Argus II FDA Approval

Nasdaq : EYES R&D: Software Upgrades 11 From Bonham and Litvak , “ Current Focusing and Steering, ” Hearing Research 242 (2008), 141 - 153. Resolution enhancement without adding electrodes • Controlling the relative stimulation applied to adjacent electrodes can produce spatial patterns of stimulation between electrodes ( “ virtual electrodes ” ) • Potential resolution enhancement: 10x – 100x at the pixel level • Large body of work to draw upon in cochlear implant technology • Capital efficient path to resolution enhancement • Key variables: • Relative pulse amplitude in adjacent electrodes • Pulse shape • Pulse duration
Nasdaq : EYES R&D: Expanding into AMD Experimental work conducted in Armand Tanguay Jr. ’ s lab at USC indicated simulations with retinal prostheses in AMD patients may: • Improve time to grasp a target object and ability to avoid obstacles to grasping a target • Improve functional depth task performance • Allow blind patients to perform daily tasks with more ease, accuracy , and speed Human experiments at Johns Hopkins produced phosphenes in two AMD patients 12 Preliminary data indicates Argus II may be an effective treatment for AMD
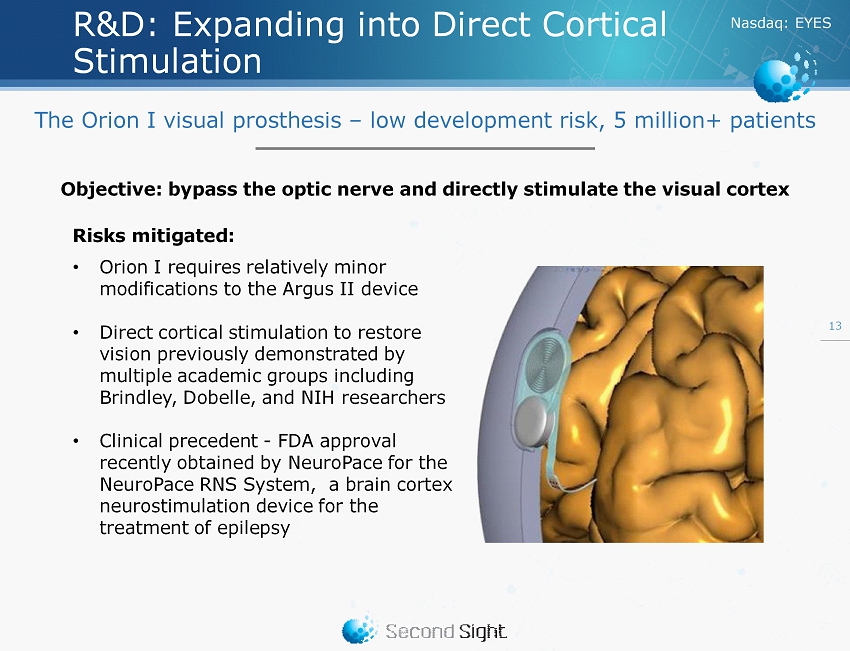
Nasdaq : EYES R&D: Expanding into Direct Cortical Stimulation 13 Objective: bypass the optic nerve and directly stimulate the visual cortex Risks mitigated: • Orion I requires relatively minor modifications to the Argus II device • Direct cortical stimulation to restore vision previously demonstrated by multiple academic groups including Brindley , Dobelle , and NIH researchers • Clinical precedent - FDA approval recently obtained by NeuroPace for the NeuroPace RNS System, a brain cortex neurostimulation device for the treatment of epilepsy The Orion I visual prosthesis – low development risk, 5 million+ patients
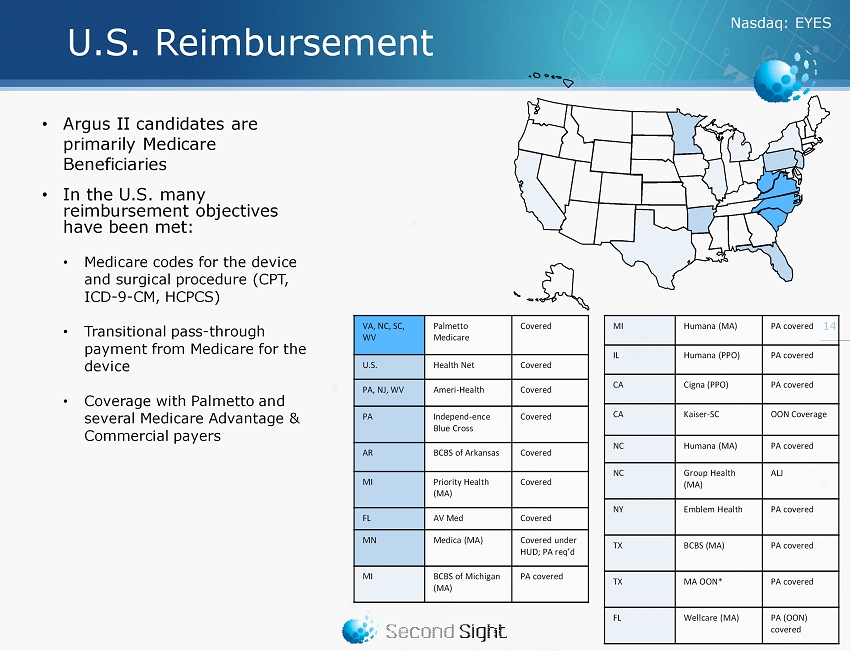
Nasdaq : EYES U.S. Reimbursement 14 • Argus II candidates are primarily Medicare Beneficiaries • In the U.S. many reimbursement objectives have been met: • Medicare codes for the device and surgical procedure (CPT, ICD - 9 - CM, HCPCS) • Transitional pass - through payment from Medicare for the device • Coverage with Palmetto and several Medicare Advantage & Commercial payers VA, NC, SC, WV Palmetto Medicare Covered U.S. Health Net Covered PA, NJ, WV Ameri - Health Covered PA Independ - ence Blue Cross Covered AR BCBS of Arkansas Covered MI Priority Health (MA) Covered FL AV Med Covered MN Medica (MA) Covered under HUD; PA req’d MI BCBS of Michigan (MA) PA covered MI Humana (MA) PA covered IL Humana (PPO) PA covered CA Cigna (PPO) PA covered CA Kaiser - SC OON Coverage NC Humana (MA) PA covered NC Group Health (MA) ALJ NY Emblem Health PA covered TX BCBS (MA) PA covered TX MA OON* PA covered FL Wellcare (MA) PA (OON) covered
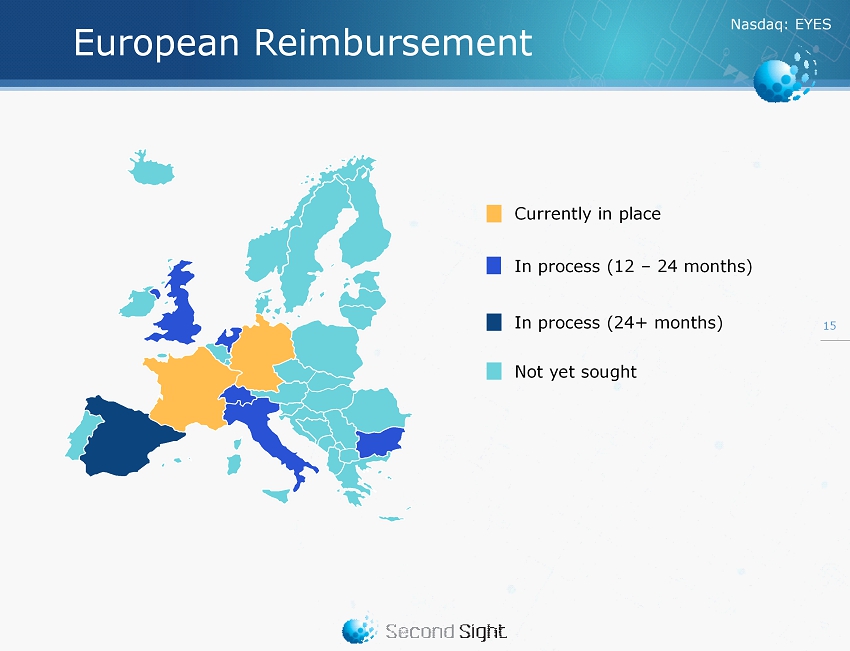
Nasdaq : EYES 15 Currently in place In process (24 + months) Not yet sought In process (12 – 24 months) European Reimbursement

Nasdaq : EYES North America Kellogg – Univ . Michigan Univ . Southern California Toronto Western Hospital Wills Eye – Philadelphia Duke Eye Center Texas Retina Assoc . – Dallas Bascom Palmer – Univ . Miami Wilmer – Johns Hopkins Emory University Univ . Illinois Chicago Mayo Clinic – Minnesota Germany University Aachen University Cologne University Hamburg University Lübeck City Karlsruhe Clinic Sulzbach Saudi Arabia King Khaled Eye Specialist Hospital Implanting Centers Worldwide 16 France CHU Bordeaux CHU Strasbourg CHNO des XV - XX (Paris) • North America – 11 centers; in discussions with 12 more • EU/Middle East – 12 centers; in discussions with 13 more • Asia partnerships anticipated as technology platform matures Italy Firenze Camposanpierro (Venice)
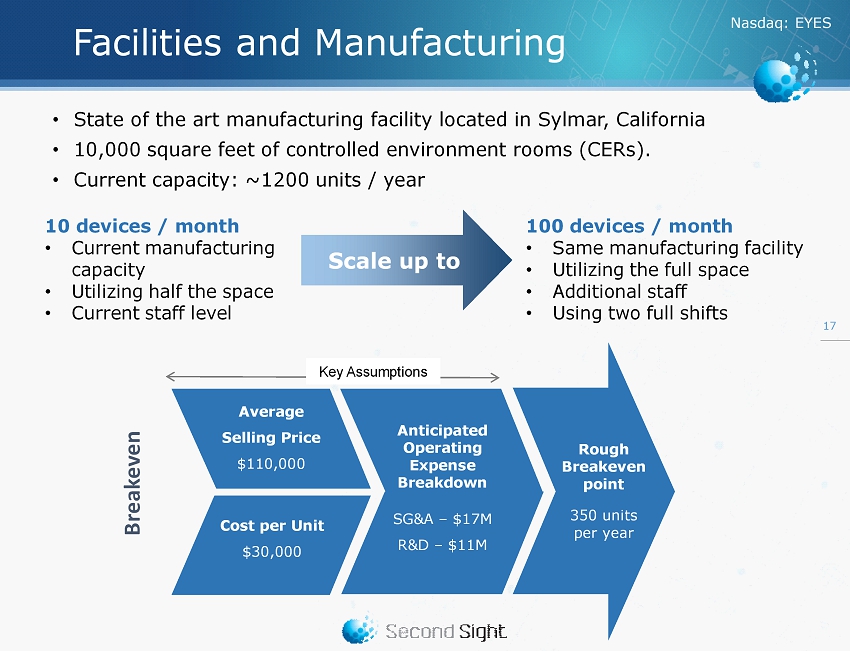
Nasdaq : EYES Facilities and Manufacturing • State of the art manufacturing facility located in Sylmar, California • 10,000 square feet of controlled environment rooms (CERs). • Current capacity: ~1200 units / year 17 10 devices / month • Current manufacturing capacity • Utilizing half the space • Current staff level 100 devices / month • Same manufacturing facility • U tilizing the full space • Additional staff • Using two full shifts Scale up to Cost per Unit $30,000 Average Selling Price $110,000 Rough Breakeven point 350 units per year Anticipated Operating Expense Breakdown SG&A – $17M R&D – $ 11M Key Assumptions Breakeven

Nasdaq : EYES Patent Protection We solved five very difficult technical problems that to our knowledge no other company has solved . Our patent portfolio contains a large number of claims covering these solutions: 18 Other challenges, 178 grants and applications Reliable long lived miniature electronics package, 83 grants and apps Safe and reliable flexible neural interface with many electrodes, 76 grants and apps Interconnect package to electrode array, 38 grants and apps Material capable of high charge injection on small electrodes, 48 grants and apps Effective stimulation patterns and fitting, 36 grants and apps USPTO Grants 219 Pending Applications 76 Foreign Offices Grants 88 Pending Applications 76 Large portfolio creates significant barriers to entry 178 grants a nd apps on other challenges 281 grants and apps on the 5 challenges
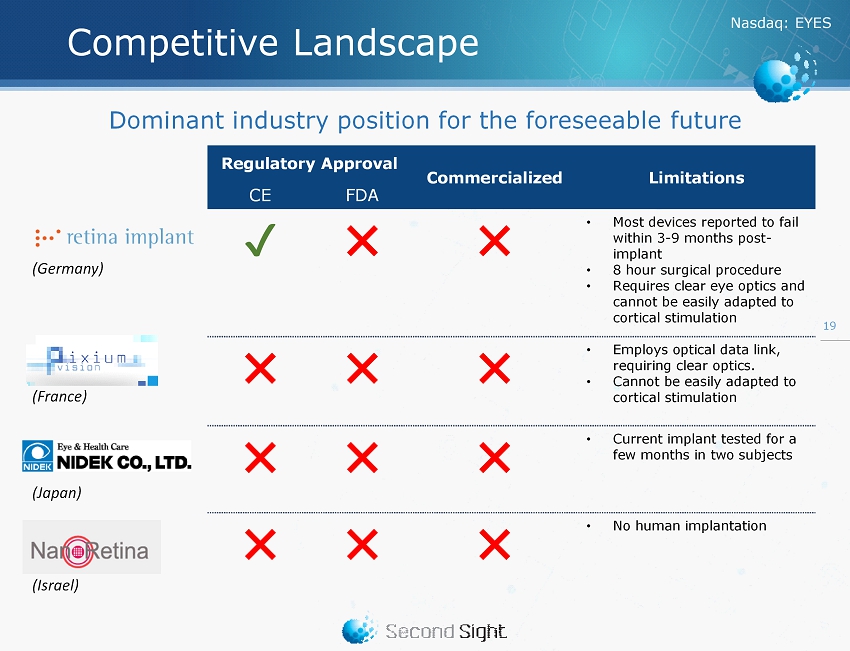
Nasdaq : EYES Competitive Landscape Regulatory Approval Commercialized Limitations CE FDA ✔ ✖ ✖ • Most devices reported to fail within 3 - 9 months post - implant • 8 hour surgical procedure • Requires clear eye optics and cannot be easily adapted to cortical stimulation ✖ ✖ ✖ • Employs optical data link, requiring clear optics. • Cannot be easily adapted to cortical stimulation ✖ ✖ ✖ • Current implant tested for a few months in two subjects ✖ ✖ ✖ • No human implantation 19 Dominant industry position for the foreseeable future (Germany) (France) (Japan) (Israel)

Nasdaq : EYES Major Awards & Recognition 20

Nasdaq : EYES 21 Investment Highlights • Restoring vision to the blind with the Argus II platform technology • Targeting significant addressable market of over 8 million people • Securing our market leading position with strong patent protection • Ensuring first mover advantage as the only device with U.S. FDA approval • Demonstrating reimbursement success in the U.S. and Europe • Enhancing financial flexibility to execute strategic objectives with successful IPO and strong balance sheet

Nasdaq : EYES Contact Retail Investor Relations Matt Hayden Chairman MZ North America Direct: 949 - 259 - 4986 Matt.Hayden@mzgroup.us Second Sight Medical Products, Inc. 12744 San Fernando Road Building 3 Sylmar, CA 91342 Direct: 818 - 833 - 5000 www.secondsight.com Institutional Investor Relations Lisa Wilson President In - Site Communications, Inc. Direct: 212 - 452 - 2793 lwilson@insitecony.com 23
Nasdaq : EYES Second Sight Medical Products Everyone Should Have Vision TM 23