
Exhibit 99.1

NASDAQ: EYES A Better Way of Life for Patients May 2017 NASDAQ: EYES

NASDAQ: EYES Forward Looking Statements This presentation contains certain forward - looking information about Second Sight that is intended to be covered by the safe harbor for "forward - looking statements" provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward - looking statements are statements that are not historical facts. Words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “strong,” “up coming,” and similar expressions are intended to identify forward - looking statements. These statements include, but are not limited to, statements regarding our ability to successfully develop and commercialize our products; our ability to expand our long - term business opportunities; financial projections and estimates and their underlying assumptions; and future performance. In this document, we refer to information regarding potential markets for products and other industry data. We believe that all such information has been obtained from reliable sources that are customarily relied upon by companies in our industry. However, we have not independently verified any such information. Forward - looking statements may address the following subjects among others: expected products, applications, customers, technologies and performance, coverage and insurance reimbursements, results of clinical studies, success of research and development and our expectations concerning our business strategy. Forward - looking statements involve inherent risks and uncertainties which could cause actual results to differ materially from those in the forward - looking statements, as a result of various factors including those risks and uncertainties referred to in the Risk Factors and in Management's Discussion and Analysis of Financial Condition and Results of Operations sections of our Annual Report on Form 10 - K as filed on March 16, 2017 and our other reports filed from time to time with the Securities and Exchange Commission. The audience is cautioned not to place undue reliance on these forward - looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward - looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non - occurrence of any events. 2

NASDAQ: EYES Second Sight Medical Products, Inc. Enabling blind individuals to achieve greater independence & quality of life with implantable visual prosthetics Our Mission
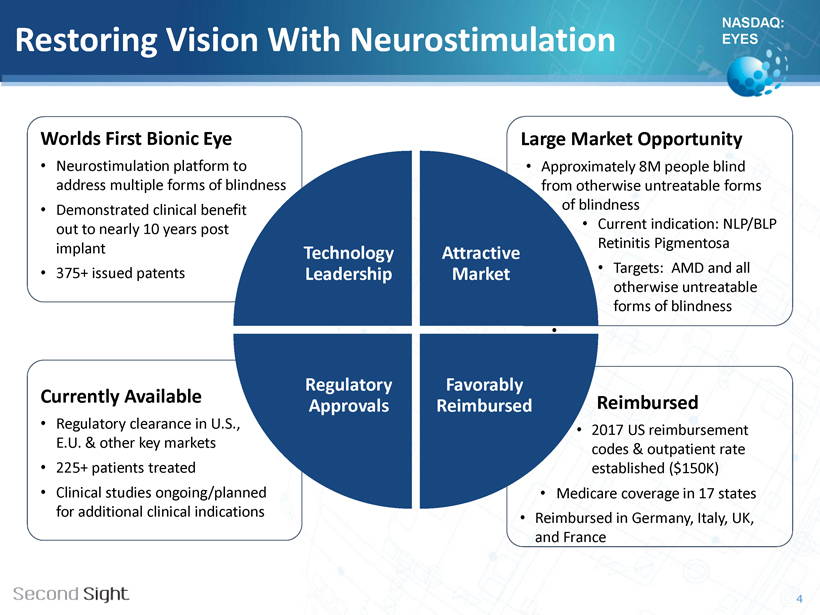
NASDAQ: EYES Restoring Vision With Neurostimulation Worlds First Bionic Eye • Neurostimulation platform to address multiple forms of blindness • Demonstrated clinical benefit out to nearly 10 years post implant • 375+ issued patents Currently Available • Regulatory clearance in U.S., EU E.U. & other key markets • 225+ patients treated • Clinical studies ongoing/planned for additional clinical indications Large Market Opportunity • Approximately 8M people blind from otherwise untreatable forms of blindness • Current indication: NLP/BLP Retinitis Pigmentosa • Targets: AMD and all otherwise untreatable forms of blindness • Reimbursed • 2017 US reimbursement codes & outpatient rate established ($150K) • Medicare coverage in 17 states • Reimbursed in Germany, Italy, UK, and France Technology Leadership Attractive Market Favorably Reimbursed Regulatory Approvals 4

NASDAQ: EYES Video: Bionic Eye Gives Sight to the Blind 5 http://www.bloomberg.com/news/videos/2015 - 05 - 21/bionic - eyes - give - second - sight - to - the - blind
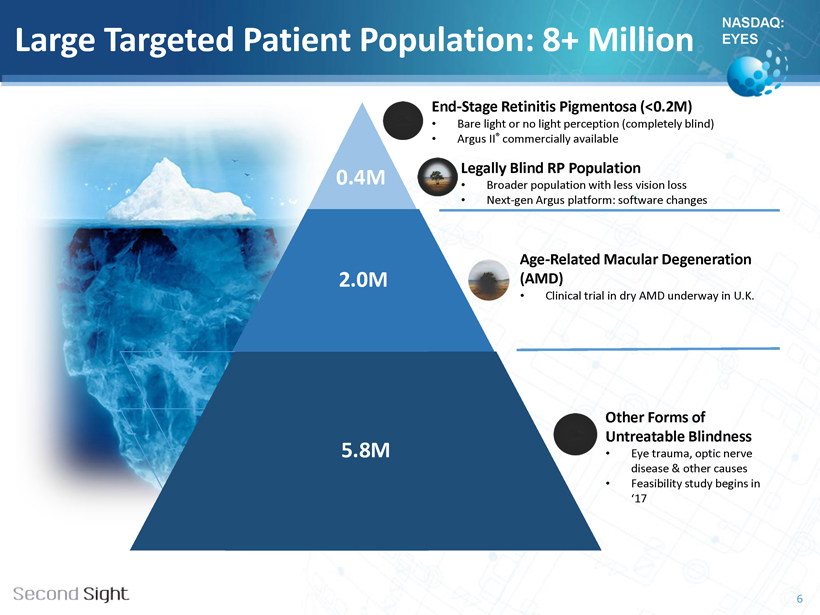
NASDAQ: EYES Large Targeted Patient Population: 8+ Million • Age - Related Macular Degeneration (AMD) • Clinical trial in dry AMD underway in U.K. 2.0M • Other forms of untreatable blindness • Eye trauma, optic nerve disease & other causes • Feasibility study begins in ‘17 5.8M 6 0.4M End - Stage Retinitis Pigmentosa (<0.2M) • Bare light or no light perception (completely blind) • Argus II ® commercially available Legally Blind RP Population • Broader population with less vision loss • Next - gen Argus platform: software changes Age - Related Macular Degeneration (AMD) • Clinical trial in dry AMD underway in U.K. Other Forms of Untreatable Blindness • Eye trauma, optic nerve disease & other causes • Feasibility study begins in ‘17
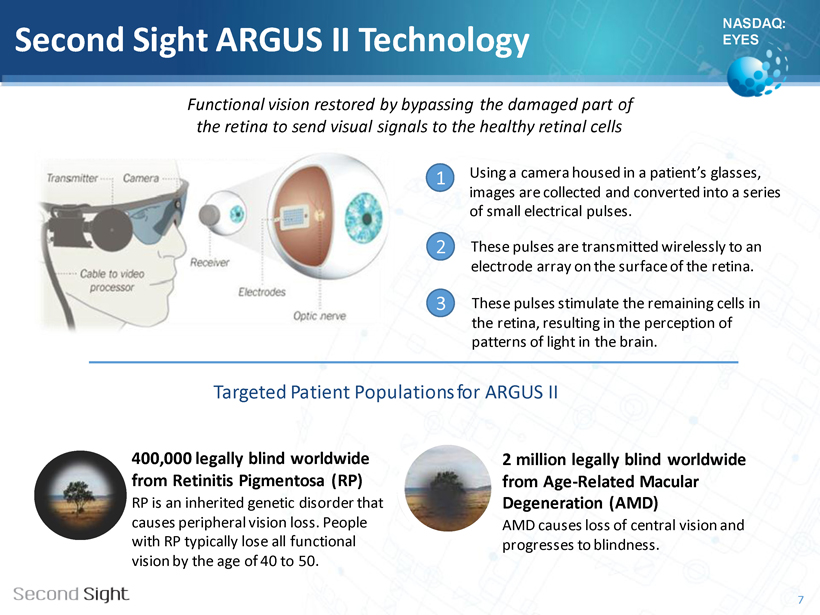
NASDAQ: EYES Second Sight ARGUS II Technology Using a camera housed in a patient’s glasses, images are collected and converted into a series of small electrical pulses. These pulses are transmitted wirelessly to an electrode array on the surface of the retina. These pulses stimulate the remaining cells in the retina, resulting in the perception of patterns of light in the brain. Targeted Patient Populations for ARGUS II 7 1 2 3 400,000 legally blind worldwide from Retinitis Pigmentosa (RP) RP is an inherited genetic disorder that causes peripheral vision loss. People with RP typically lose all functional vision between the ages of 40 to 50. 2 million legally blind worldwide from Age - Related Macular Degeneration (AMD) AMD causes loss of central vision and progresses to blindness. Functional vision restored by bypassing the damaged part of the retina to send visual signals to the healthy retinal cells
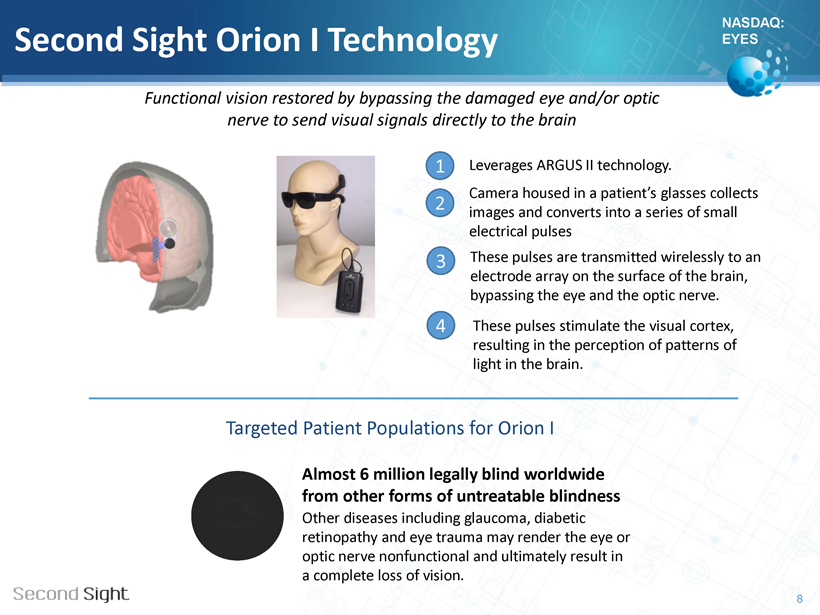
NASDAQ: EYES Second Sight Orion I Technology Leverages ARGUS II technology. Camera housed in a patient’s glasses collects images and converts into a series of small electrical pulses These pulses are transmitted wirelessly to an electrode array on the surface of the brain, bypassing the eye and the optic nerve. Targeted Patient Populations for Orion I 8 1 2 3 Almost 6 million legally blind worldwide from other forms of untreatable blindness Other diseases including glaucoma, diabetic retinopathy and eye trauma may render the eye or optic nerve nonfunctional and ultimately result in a complete loss of vision. 4 These pulses stimulate the visual cortex, resulting in the perception of patterns of light in the brain. Functional vision restored by bypassing the damaged eye and/or optic nerve to send visual signals directly to the brain

NASDAQ: EYES Established Technology Leadership 9 • Over 375 patents issued • Over 125 patents pending Pioneering Technology Groundbreaking Research Recognized Leadership • 75+ scientific publications • 6 clinical & post - approval studies • Demonstrated benefit out to ~10 years post - implant • Numerous innovation awards • Coverage in all major countries and US media markets The 50 Smartest Companies for 2014
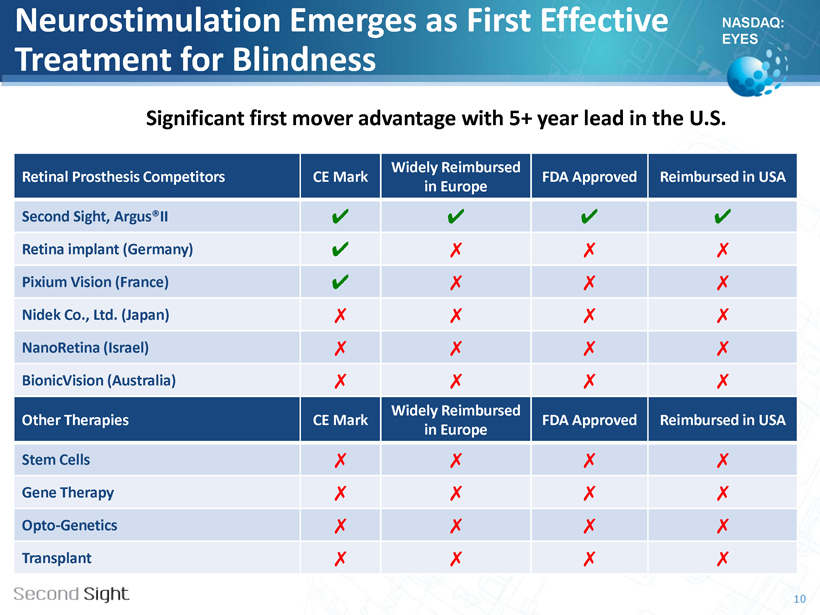
NASDAQ: EYES Neurostimulation Emerges as First Effective Treatment for Blindness 10 Retinal Prosthesis Competitors CE Mark Widely Reimbursed in Europe FDA Approved Reimbursed in USA Second Sight, Argus®II ✔ ✔ ✔ ✔ Retina implant (Germany) ✔ ✗ ✗ ✗ Pixium Vision (France) ✔ ✗ ✗ ✗ Nidek Co., Ltd. (Japan) ✗ ✗ ✗ ✗ NanoRetina (Israel) ✗ ✗ ✗ ✗ BionicVision (Australia) ✗ ✗ ✗ ✗ Other Therapies CE Mark Widely Reimbursed in Europe FDA Approved Reimbursed in USA Stem Cells ✗ ✗ ✗ ✗ Gene Therapy ✗ ✗ ✗ ✗ Opto - Genetics ✗ ✗ ✗ ✗ Transplant ✗ ✗ ✗ ✗ Significant first mover advantage with 5+ year lead in the U.S.
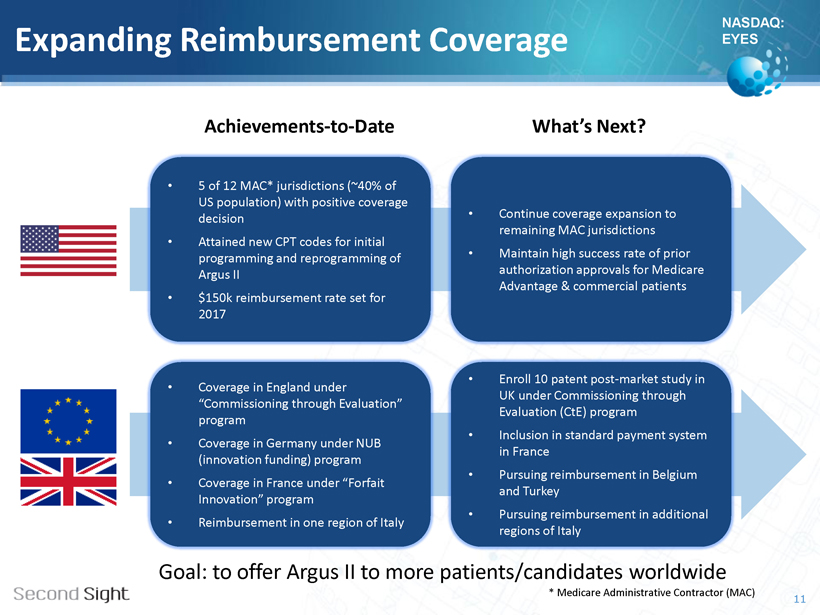
NASDAQ: EYES Expanding Reimbursement Coverage 11 • 5 of 12 MAC* jurisdictions (~40% of US population) with positive coverage decision • Attained new CPT codes for initial programming and reprogramming of Argus II • $150k reimbursement rate set for 2017 • Continue coverage expansion to remaining MAC jurisdictions • Maintain high success rate of prior authorization approvals for Medicare Advantage & commercial patients Achievements - to - Date What’s Next? • Coverage in England under “Commissioning through Evaluation” program • Coverage in Germany under NUB (innovation funding) program • Coverage in France under “ Forfait Innovation” program • Reimbursement in one region of Italy • Enroll 10 patent post - market study in UK under Commissioning through Evaluation ( CtE ) program • Inclusion in standard payment system in France • Pursuing reimbursement in Belgium and Turkey • Pursuing reimbursement in additional regions of Italy * Medicare Administrative Contractor (MAC) Goal: to offer Argus II to more patients/candidates worldwide
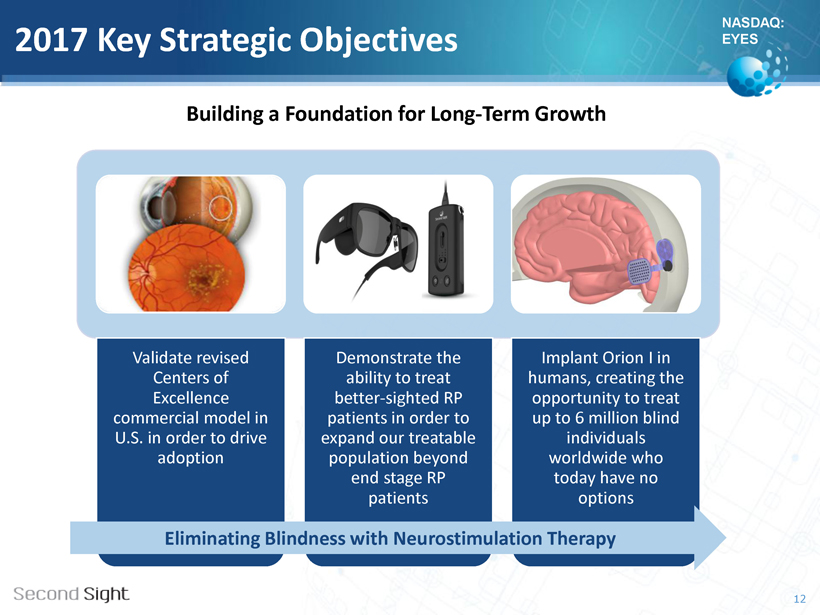
NASDAQ: EYES 2017 Key Strategic Objectives Validate revised Centers of Excellence commercial model in U.S. in order to drive adoption Demonstrate the ability to treat better - sighted RP patients in order to expand our treatable population beyond end stage RP patients Implant Orion I in humans, creating the opportunity to treat up to 6 million blind individuals worldwide who today have no options Eliminating Blindness with Neurostimulation Therapy 12 Building a Foundation for Long - Term Growth
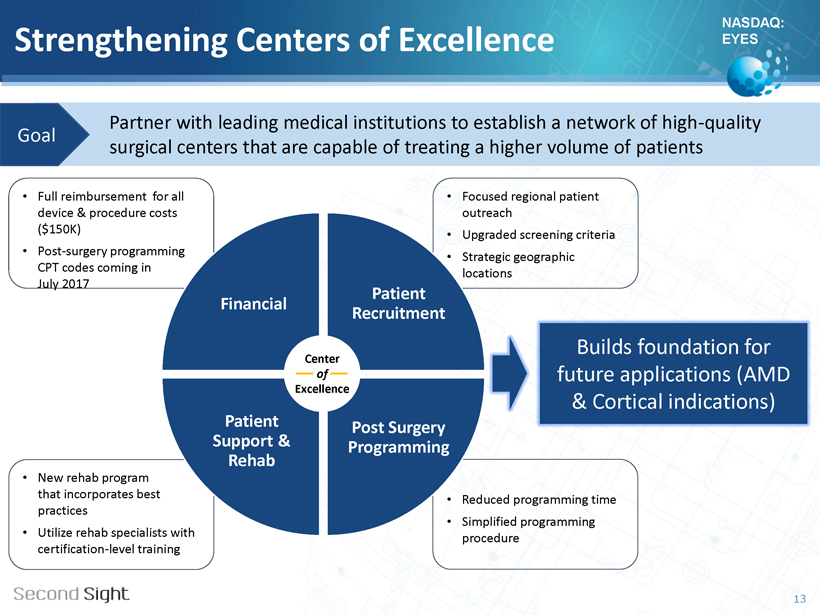
NASDAQ: EYES Strengthening Centers of Excellence Partner with leading medical institutions to establish a network of high - quality surgical centers that are capable of treating a higher volume of patients Goal • New rehab program that incorporates best practices • Utilize rehab specialists with certification - level training • Full reimbursement for all device & procedure costs ($150K) • Post - surgery programming CPT codes coming in July 2017 • Focused regional patient outreach • Upgraded screening criteria • Strategic geographic locations • Reduced programming time • Simplified programming procedure 13 Builds foundation for future applications (AMD & Cortical indications) Financial Patient Recruitment Post Surgery Programming Patient Support & Rehab Center of Excellence
NASDAQ: EYES Treating Better - Sighted Patients with Argus II Enhancements 14 Clinical Testing of Current Argus II Technology in Better - Sighted RP Patients Clinical Testing of Argus II Enhancements in Better - Sighted RP & AMD Patients • New external components for streamlined appearance • Software improvements for advanced image processing • Improved retinal stimulation
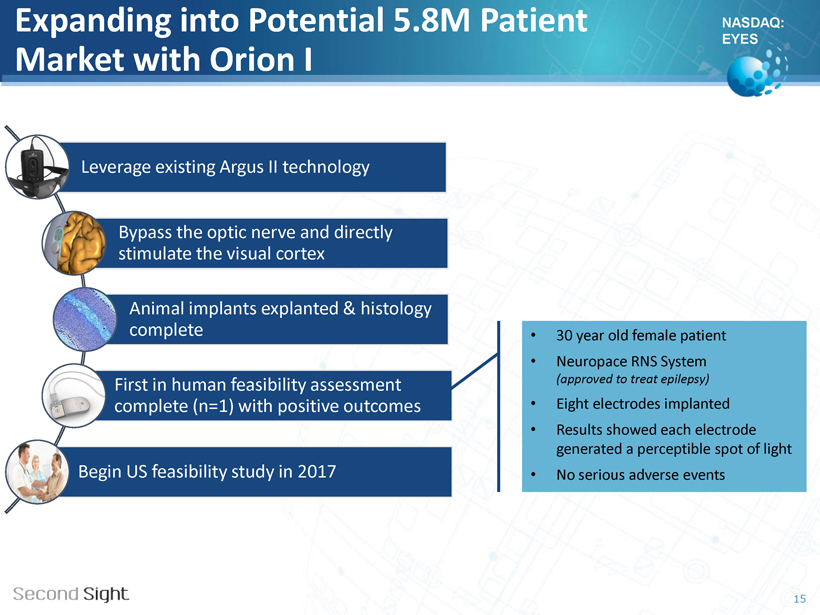
NASDAQ: EYES Expanding into Potential 5.8M Patient Market with Orion I Leverage existing Argus II technology Bypass the optic nerve and directly stimulate the visual cortex Animal implants explanted & histology complete First in human feasibility assessment complete (n=1) with positive outcomes Begin US feasibility study in 2017 • 30 year old female patient • Neuropace RNS System (approved to treat epilepsy) • Eight electrodes implanted • Results showed each electrode generated a perceptible spot of light • No serious adverse events 15
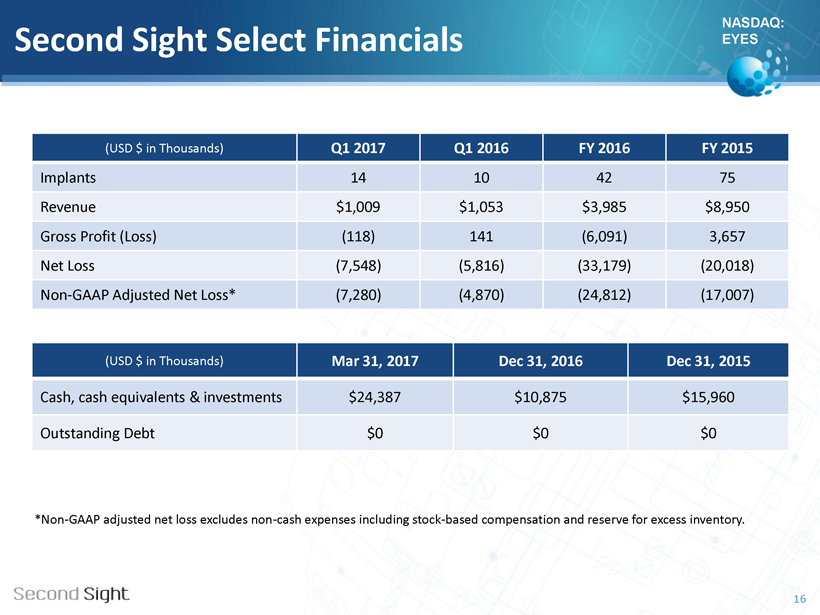
NASDAQ: EYES Second Sight Select Financials 16 *Non - GAAP adjusted net loss excludes non - cash expenses including stock - based compensation and reserve for excess inventory. (USD $ in Thousands) Q1 2017 Q1 2016 FY 2016 FY 2015 Implants 14 10 42 75 Revenue $1,009 $1,053 $3,985 $8,950 Gross Profit (Loss) (118) 141 (6,091) 3,657 Net L oss (7,548) (5,816) (33,179) (20,018) Non - GAAP Adjusted Net Loss* (7,280) (4,870) (24,812) (17,007) (USD $ in Thousands) Mar 31 , 2017 Dec 31, 2016 Dec 31, 2015 Cash, cash equivalents & investments $24,387 $10,875 $15,960 Outstanding Debt $0 $0 $0
NASDAQ: EYES A Better Way of Life for Patients 17 Improved Orientation and Mobility Greater Independence Significant Improvement in Quality of Life Enhanced Connection to Loved Ones

NASDAQ: EYES Second Sight Investment Highlights Technology Leadership • First and only treatment approved for sale in the US, EU, Saudi Arabia, Turkey and Canada • Robust IP portfolio with 500+ patents issued and pending • Significant first mover advantage with 5+ year lead over any other therapeutic option in US Large Market Opportunity • RP, AMD and other untreatable forms of blindness totaling an estimated 8 - plus million people • Significant unmet clinical need with proven clinical benefit Increased Adoption of Argus II in 2017 • Centers of Excellence commercial model • Technology enhancements to broaden RP target to better - sighted patients Established Reimbursement in US and Europe • 2017 established rate of $150k in US with coverage in 17 states • CPT codes to be established (July 1, 2017) for post - surgery programming • Reimbursed in Germany, Italy, France and UK Robust R&D pipeline • Argus II improvements to advance performance and expand markets • Orion I for nearly all untreatable forms of blindness ready for clinical study in 2017 18

NASDAQ: EYES Retail Investor Relations Greg Falesnik Managing Director MZ North America Direct: 949 - 385 - 6449 Greg.Falesnik@mzgroup.us Second Sight Medical Products, Inc. 12744 San Fernando Road Suite 400 Sylmar, CA 91342 Main: 818 - 833 - 5000 www.secondsight.com Institutional Investor Relations Lisa Wilson President In - Site Communications, Inc. Direct: 212 - 452 - 2793 lwilson@insitecony.com Contacts Will McGuire President & CEO Direct: 818 - 833 - 5040 wmcguire@secondsight.com 19