
Exhibit 99.1

NASDAQ: EYES Enriching the Lives of the Blind May 2018 NASDAQ: EYES

NASDAQ: EYES Forward Looking Statements This press release contains forward - looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange and Exchange Act of 1934, as amended, which are intended to be covered by the "safe harbor" created by those sections. All statements in this release that are not based on historical fact are "forward looking statements." These statements may be identified by words such as "estimates," "anticipates," "projects," "plans," or "planned," "seeks," "may," "will," "expects," "intends," "believes," "should," and similar expressions, or the negative versions thereof, and which also may be identified by their context. All statements that address operating performance or events or developments that Second Sight expects or anticipates will occur in the future, such as stated objectives or goals, or that are not otherwise historical facts, are forward - looking statements. While management has based any forward - looking statements included in this release on its current expectations, the information on which such expectations were based may change. Forward - looking statements involve inherent risks and uncertainties which could cause actual results to differ materially from those in the forward - looking statements, as a result of various factors including those risks and uncertainties described in the Risk Factors and in Management's Discussion and Analysis of Financial Condition and Results of Operations sections of our Annual Report, on Form 10 - K, filed on March 20, 2018, and our other reports filed from time to time with the Securities and Exchange Commission. We urge you to consider those risks and uncertainties in evaluating our forward - looking statements. We caution readers not to place undue reliance upon any such forward - looking statements, which speak only as of the date made. Except as otherwise required by the federal securities laws, we disclaim any obligation or undertaking to publicly release any updates or revisions to any forward - looking statement contained herein (or elsewhere) to reflect any change in our expectations with regard thereto, or any change in events, conditions, or circumstances on which any such statement is based. 2
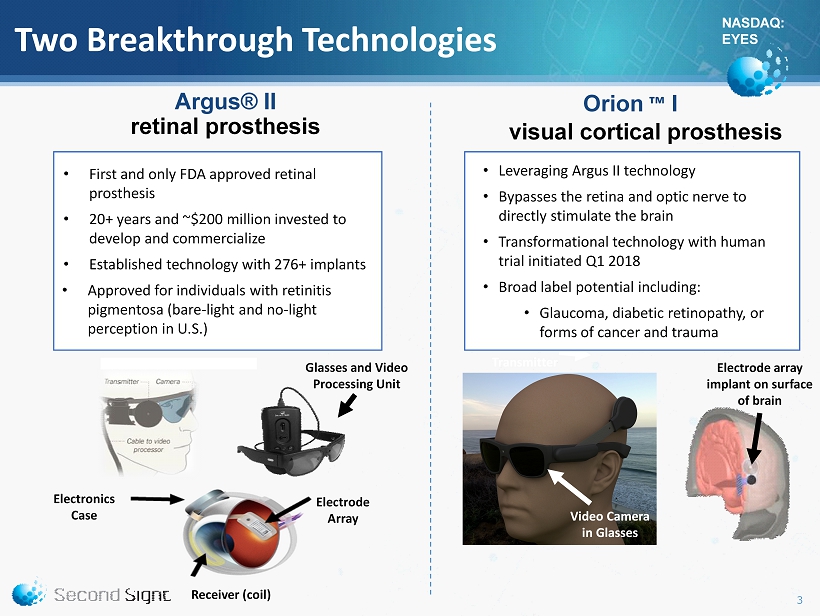
NASDAQ: EYES Two Breakthrough Technologies Argus® II retinal prosthesis 3 Glasses and Video Processing Unit Electronics Case Electrode Array Receiver (coil) Electrode array implant on surface of brain Video Camera in Glasses Wireless Transmitter Orion ™ I visual cortical prosthesis system • First and only FDA approved retinal prosthesis • 20+ years and ~$200 million invested to develop and commercialize • Established technology with 276+ implants • Approved for individuals with retinitis pigmentosa (bare - light and no - light perception in U.S.) • Leveraging Argus II technology • Bypasses the retina and optic nerve to directly stimulate the brain • Transformational technology with human trial initiated Q1 2018 • Broad label potential including: • Glaucoma, diabetic retinopathy, or forms of cancer and trauma

NASDAQ: EYES Argus® II: Effective Treatment for Blindness Argus II is a retinal prosthesis that induces visual perception in individuals with severe to profound retinitis pigmentosa (RP) 4

NASDAQ: EYES Orion I: Breakthrough Device Leveraging Argus II technology, our breakthrough Orion platform bypasses the damaged eye and/or optic nerve to directly stimulate the brain • Orion has the potential to treat many forms of blindness including glaucoma, diabetic retinopathy, forms of cancer and trauma • Received FDA Breakthrough Device designation • Completed initial FDA submission in 2018 • Requested FDA meeting to discuss regulatory and clinical path forward • Conducting a five subject feasibility study at UCLA Medical Center and the Baylor College of Medicine in Houston • First two human subjects implanted and activated*; phase 2 spatial mapping underway • Four of five subjects implanted with fifth planned for Q2 2018 • No serious adverse events reported 5 * As of May 10, 2018
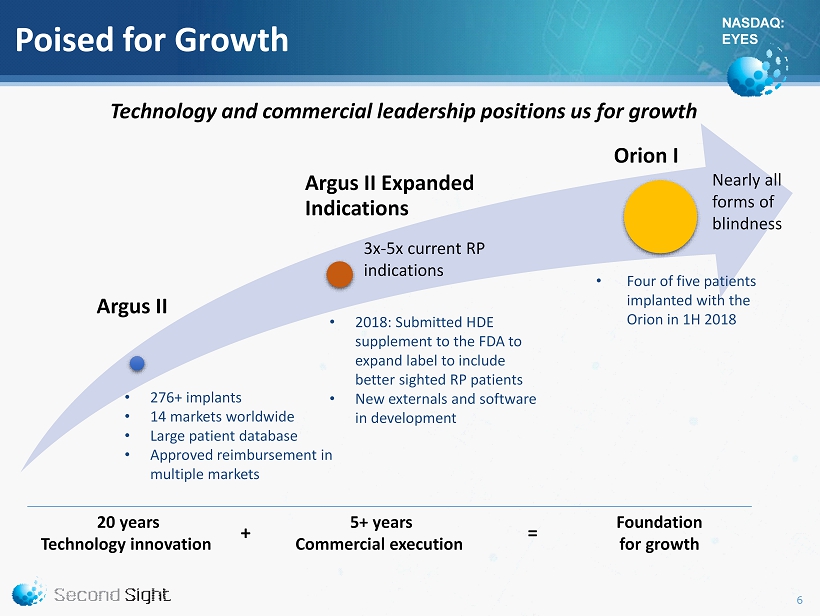
NASDAQ: EYES Poised for Growth 20 years Technology innovation 5+ years Commercial execution + Argus II Argus II Expanded Indications Orion I • 276+ implants • 14 markets worldwide • Large patient database • Approved reimbursement in multiple markets Foundation for growth = • 2018: Submitted HDE supplement to the FDA to expand label to include better sighted RP patients • New externals and software in development • Four of five patients implanted with the Orion in 1H 2018 3x - 5x current RP indications Nearly all forms of blindness Technology and commercial leadership positions us for growth 6
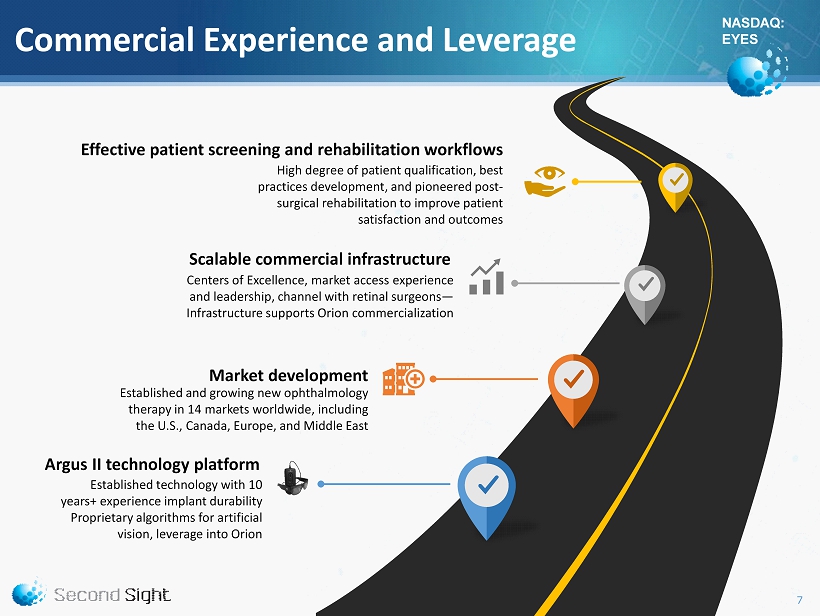
NASDAQ: EYES Commercial Experience and Leverage Established technology with 10 years+ experience implant durability Proprietary algorithms for artificial vision, leverage into Orion Argus II technology platform Established and growing new ophthalmology therapy in 14 markets worldwide, including the U.S., Canada, Europe, and Middle East Market development Centers of Excellence, market access experience and leadership, channel with retinal surgeons — Infrastructure supports Orion commercialization Scalable commercial infrastructure High degree of patient qualification, best practices development, and pioneered post - surgical rehabilitation to improve patient satisfaction and outcomes Effective patient screening and rehabilitation workflows 7
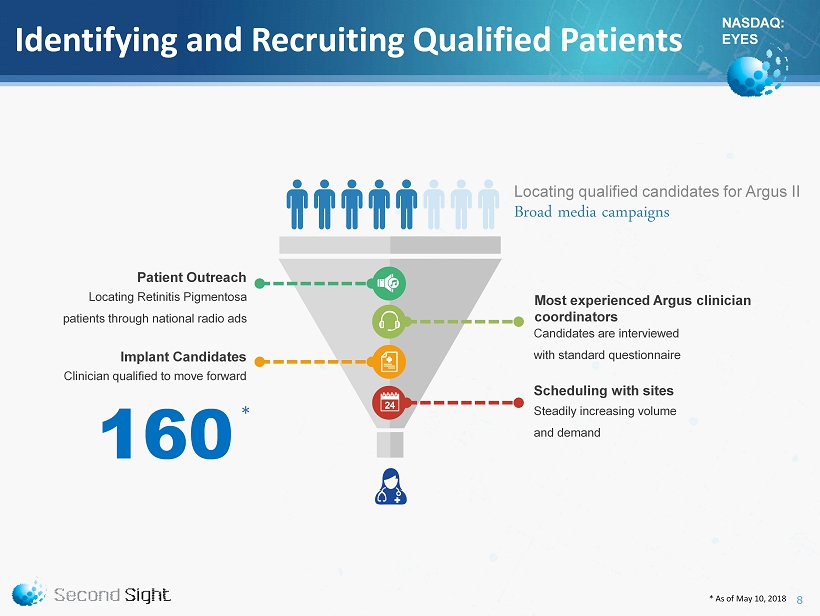
NASDAQ: EYES Identifying and Recruiting Qualified Patients Broad media campaigns Locating qualified candidates for Argus II Most experienced Argus clinician coordinators Candidates are interviewed with standard questionnaire Scheduling with sites Steadily increasing volume and demand Patient Outreach Locating Retinitis Pigmentosa patients through national radio ads Implant Candidates Clinician qualified to move forward 160 * * As of May 10, 2018 8
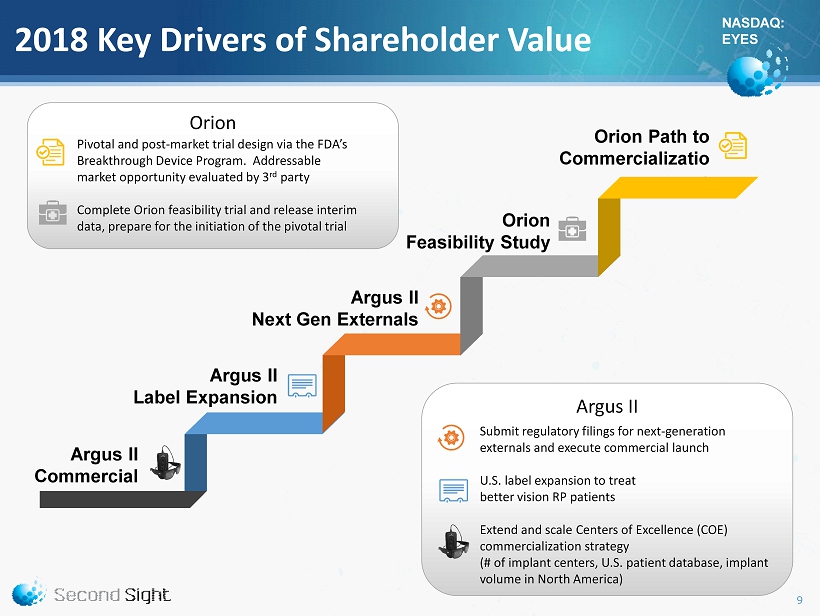
NASDAQ: EYES Orion Argus II Orion Path to Commercializatio n Argus II Commercial 2018 Key Drivers of Shareholder Value Orion Feasibility Study Pivotal and post - market trial design via the FDA’s Breakthrough Device Program. Addressable market opportunity evaluated by 3 rd party Complete Orion feasibility trial and release interim data, prepare for the initiation of the pivotal trial Argus II Label Expansion Submit regulatory filings for next - generation externals and execute commercial launch U.S. label expansion to treat better vision RP patients Extend and scale Centers of Excellence (COE) commercialization strategy (# of implant centers, U.S. patient database, implant volume in North America) Argus II Next Gen Externals 9
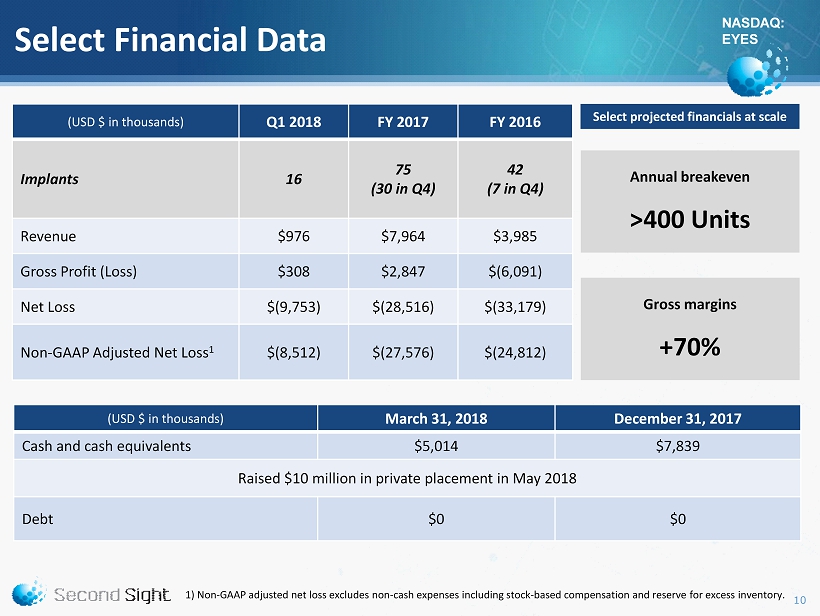
NASDAQ: EYES Select Financial Data 10 1) Non - GAAP adjusted net loss excludes non - cash expenses including stock - based compensation and reserve for excess inventory. (USD $ in thousands) Q1 2018 FY 2017 FY 2016 Implants 16 75 (30 in Q4) 42 (7 in Q4) Revenue $976 $7,964 $3,985 Gross Profit (Loss) $308 $2,847 $(6,091) Net L oss $(9,753) $(28,516) $(33,179) Non - GAAP Adjusted Net Loss 1 $(8,512) $(27,576) $(24,812) (USD $ in thousands) March 31, 2018 December 31, 2017 Cash and cash equivalents $5,014 $7,839 Raised $10 million in private placement in May 2018 Debt $0 $0 Select projected financials at scale Annual breakeven >400 Units Gross margins +70%

NASDAQ: EYES Second Sight Investment Highlights • Technology Innovator with two platforms Argus II retinal prosthesis • First and only FDA approved retinal prosthesis; 5+ year first mover advantage in the U.S. • 20 years and ~$200 million invested in established technology Orion I visual cortical prosthesis system • Leveraging Argus II technology to directly stimulate the brain • Human trial initiated Q1 2018 • Large & Expanding Addressable Market • Argus II: Currently RP with bare - light or no - light perception in U.S. • Argus II Better Vision: 3 - 5x current addressable market • Orion I: Nearly all forms of blindness including glaucoma, diabetic retinopathy, or forms of cancer and trauma • Established Argus II Reimbursement • Currently the ONLY reimbursed retinal prosthesis treating blindness in U.S. • Reimbursement or funding in Canada, Germany, Italy, France and England • R&D Pipeline Expanding Market Potential • Next generation Argus II externals and advanced stimulation programs in development • Submitted HDE supplement to the FDA during 2018 to expand Argus II label • Four of five patients implanted with the Orion in 1H 2018 11

NASDAQ: EYES Contacts Retail Investor Relations Greg Falesnik Managing Director MZ North America Direct: 949 - 385 - 6449 Greg.Falesnik@mzgroup.us 12 Second Sight Medical Products, Inc. 12744 San Fernando Road Suite 400 Sylmar, CA 91342 Main: 818 - 833 - 5000 www.secondsight.com Institutional Investor Relations Lisa Wilson President In - Site Communications, Inc. Direct: 212 - 452 - 2793 lwilson@insitecony.com Will McGuire President & CEO Direct: 818 - 833 - 5040 wmcguire@secondsight.com