Disclaimers The following slides and any accompanying oral presentation contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are intended to be covered by the "safe harbor" created by those sections. All statements in this release that are not based on historical fact are "forward looking statements." These statements may be identified by words such as "estimates," "anticipates," "projects," "plans" or "planned," "strategy," “goal," "seeks," "may," "will," "expects," "intends," "believes," "should," and similar expressions, or the negative versions thereof, and which also may be identified by their context. All statements that address operating performance or events or developments that Vivani Medical, Inc. ("Vivani", the "Company", "we" or "us) expects or anticipates will occur in the future, such as stated objectives or goals, our products and their therapeutic potential and planned development, the indications that we intend to target, our technology, our business and strategy, milestones, addressable markets, or that are not otherwise historical facts, are forward-looking statements. While management has based any forward-looking statements included in this presentation on its current expectations, the information on which such expectations were based may change. Forward-looking statements involve inherent risks and uncertainties which could cause actual results to differ materially from those in the forward-looking statements as a result of various factors. These risks and uncertainties include, but are not limited to, that we may fail to complete any required pre-clinical activities for NPM-115, NPM-119, or otherwise commence our planned clinical trials for these products under development; conduct any pre-clinical activities of our other products; our products may not demonstrate safety or efficacy in clinical trials; we may fail to secure marketing approvals for our products; there may be delays in regulatory approval or changes in regulatory framework that are out of our control; our estimation of addressable
Vivani Executive Leadership Team Adam Mendelsohn PhD – CEO/Director Co-founder/Co-inventor of Vivani technology
Vivani Medical, Inc. An innovative, biopharmaceutical company developing a portfolio of ultra long-acting, miniature, drug implants to treat chronic diseases. NanoPortal™ platform technology enables the design of implants aimed at improving medication non-adherence and tolerability.
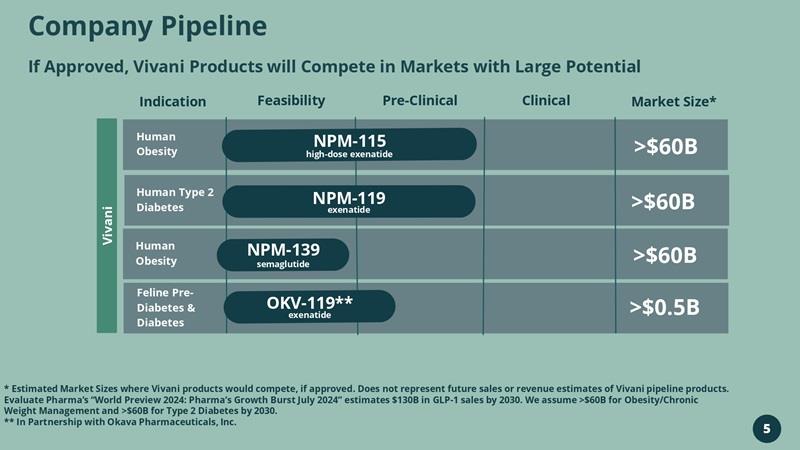
Company Pipeline If Approved, Vivani Products will Compete in Markets with Large Potential
Drug Implants Proprietary Platform Technology
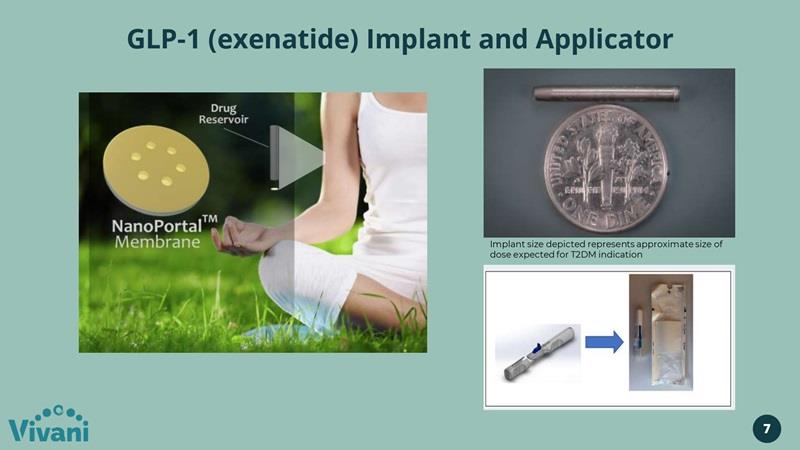
GLP-1 (exenatide) Implant and Applicator
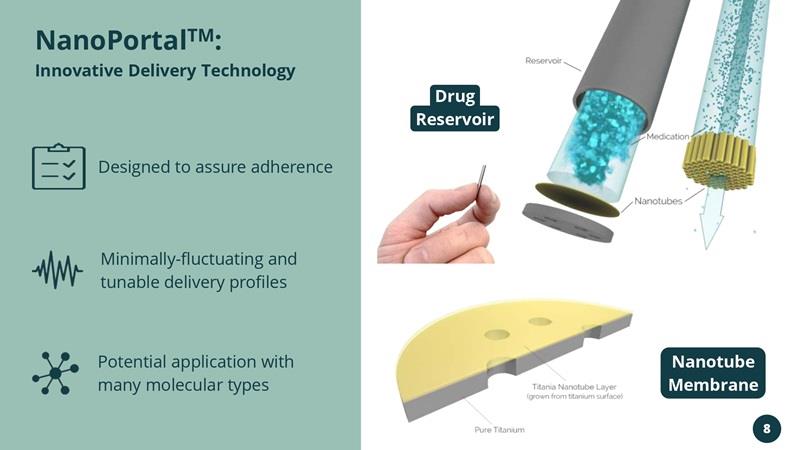
NanoPortalTM:Innovative Delivery Technology Designed to assure adherence
By precisely adjusting nanotubes to molecule size, interactions between drug and nanotube walls can result in desirable release profiles over time, including near constant release
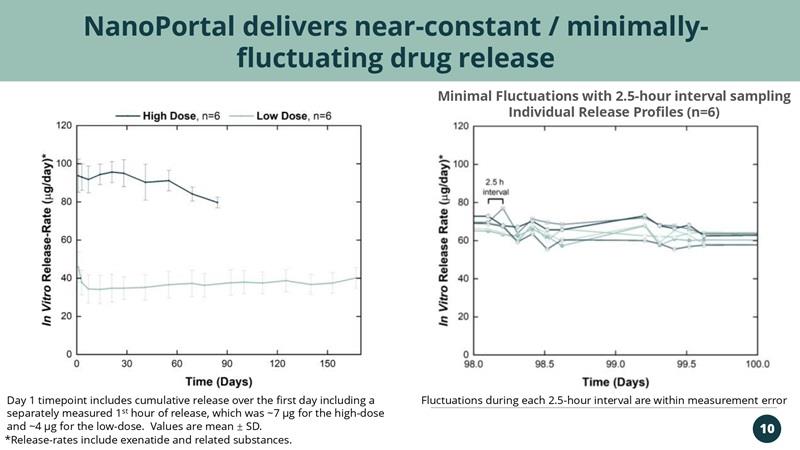
NanoPortal delivers near-constant / minimally-fluctuating drug release
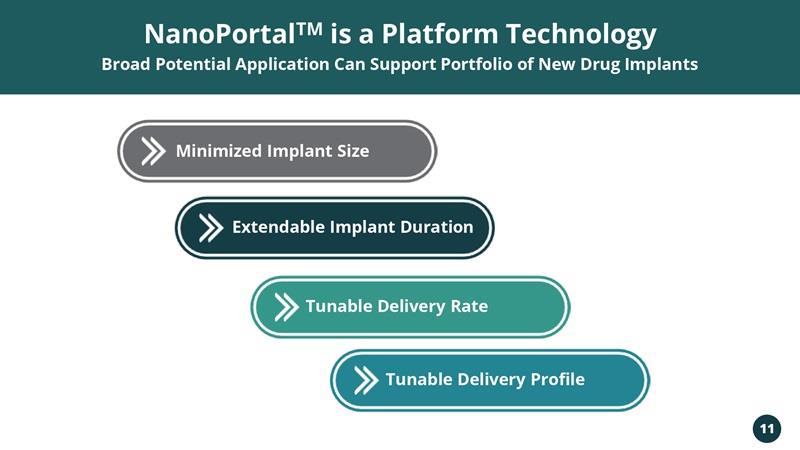
NanoPortalTM is a Platform Technology Broad Potential Application Can Support Portfolio of New Drug Implants
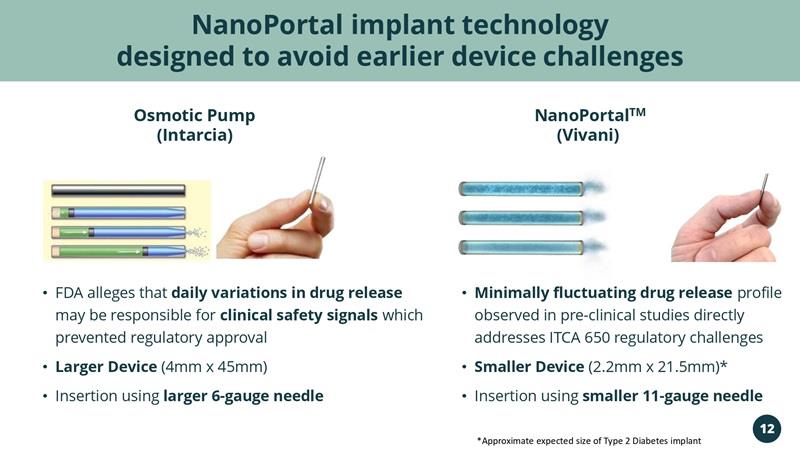
NanoPortal implant technology designed to avoid earlier device challenges
Vivani Lead Program NPM-115 High-Dose Exenatide Implant for Chronic Weight Management

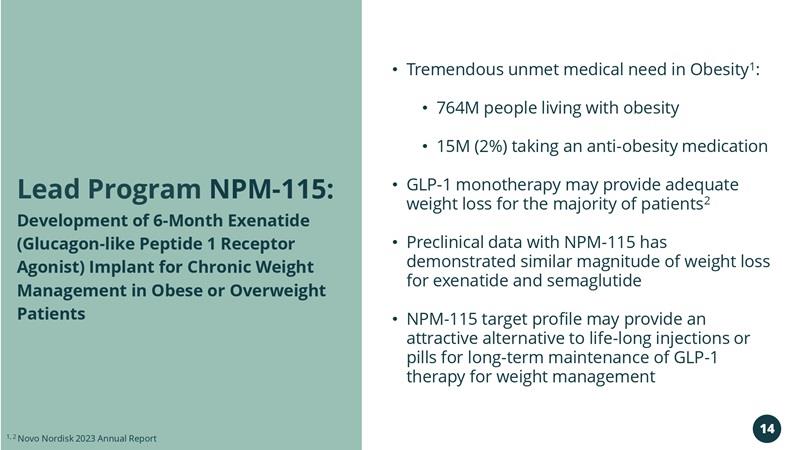
Lead Program NPM-115: Development of 6-Month Exenatide (Glucagon-like Peptide 1 Receptor Agonist) Implant for Chronic Weight Management in Obese or Overweight Patients
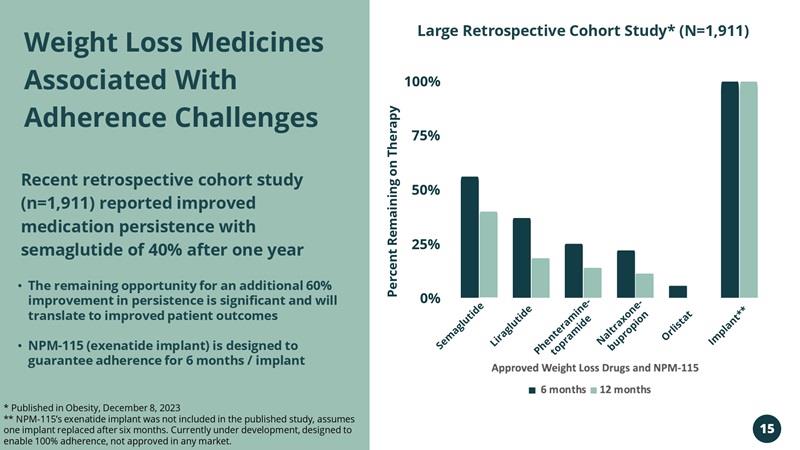
Weight Loss Medicines Associated With Adherence Challenges Recent retrospective cohort study (n=1,911) reported improved medication persistence with semaglutide of 40% after one year
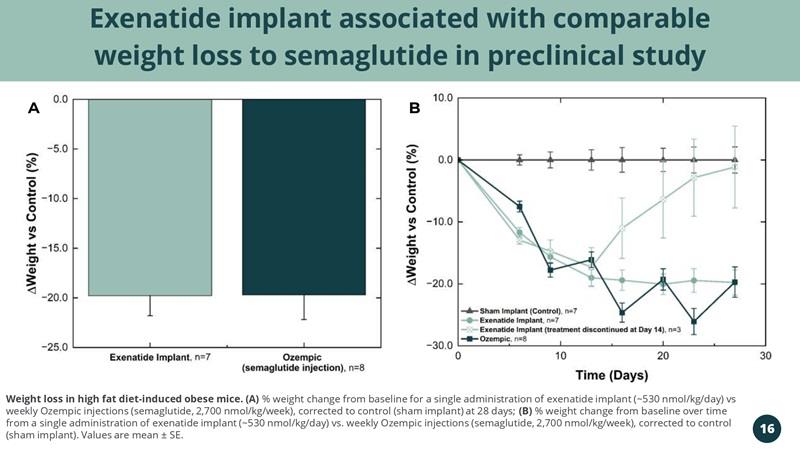
Exenatide implant associated with comparable weight loss to semaglutide in preclinical study
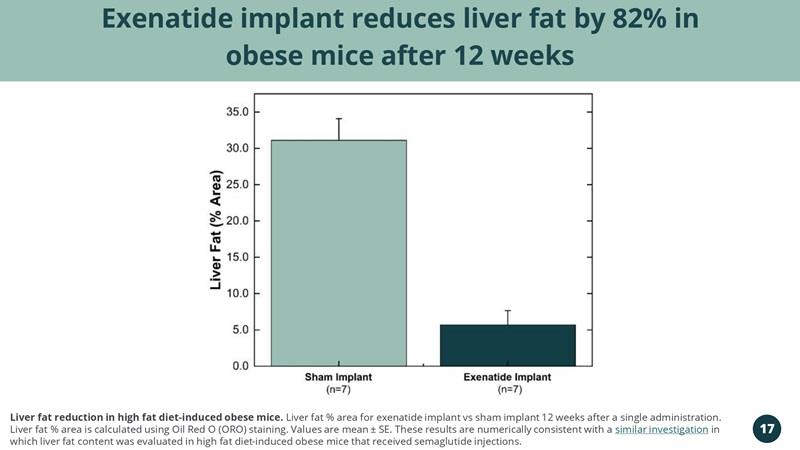
Exenatide implant reduces liver fat by 82% in obese mice after 12 weeks
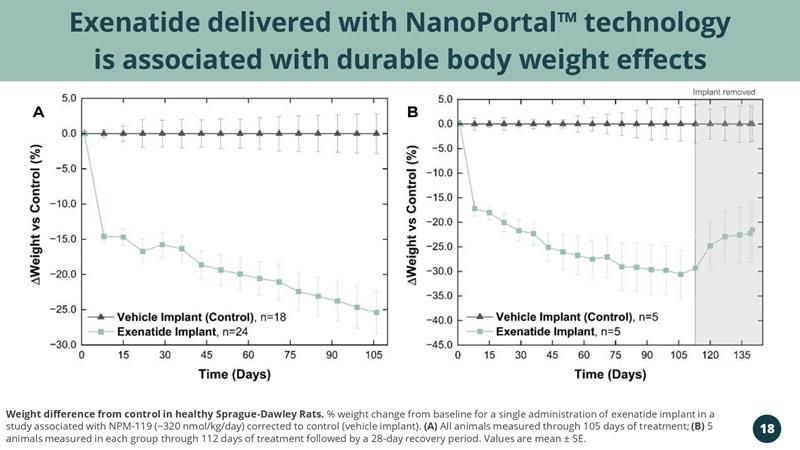
Exenatide delivered with NanoPortal™ technology is associated with durable body weight effects
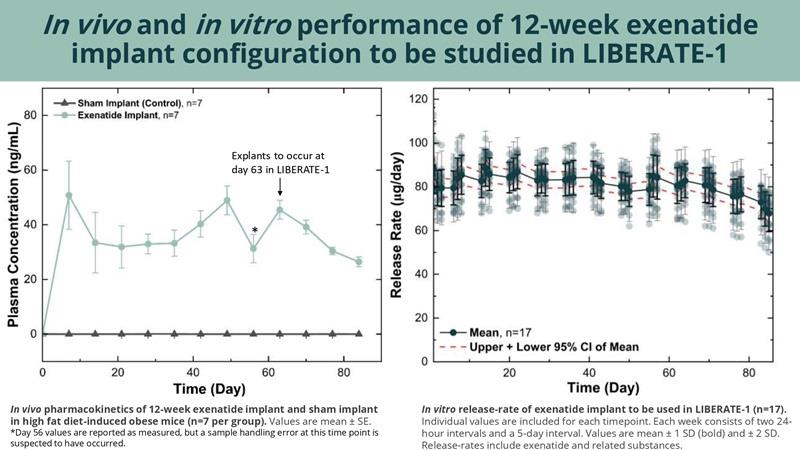
In vivo and in vitro performance of 12-week exenatide implant configuration to be studied in LIBERATE-1
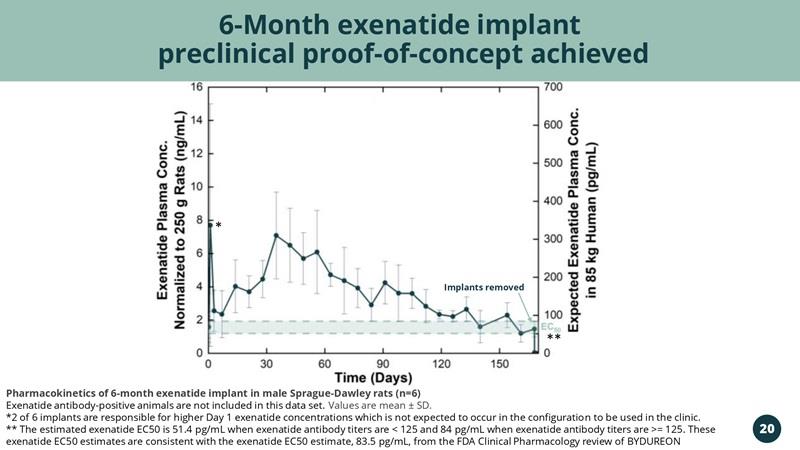
6-Month exenatide implant preclinical proof-of-concept achieved
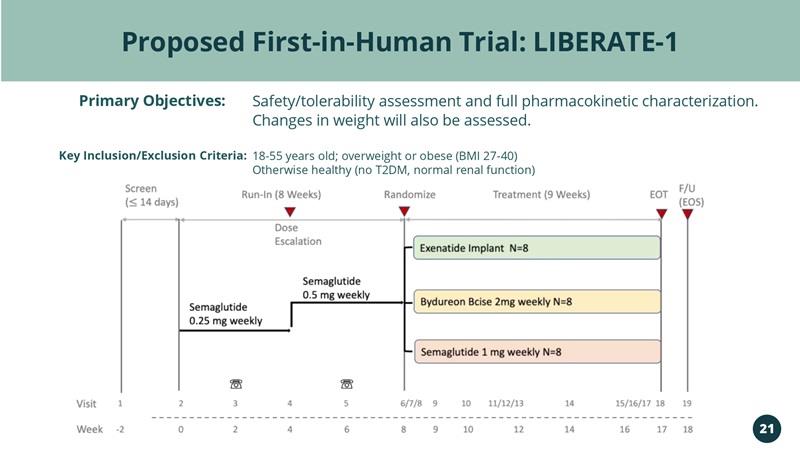
Proposed First-in-Human Trial: LIBERATE-1 Primary Objectives:
NPM-115 Clinical + Regulatory Development Near-Term Plan

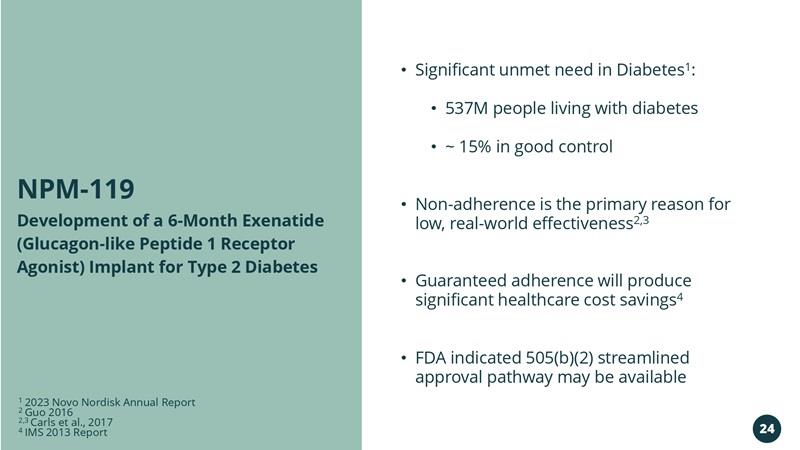
NPM-119 Exenatide Implant for Type 2 Diabetes

Development of a 6-Month Exenatide (Glucagon-like Peptide 1 Receptor Agonist) Implant for Type 2 Diabetes
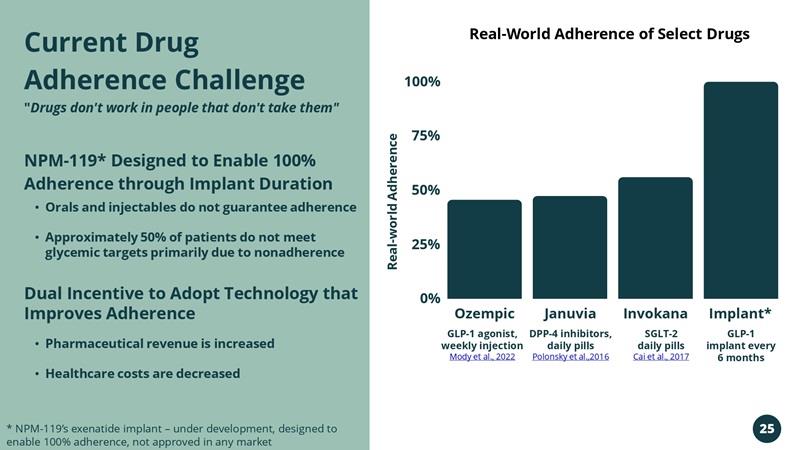
Current Drug Adherence Challenge "Drugs don't work in people that don't take them"
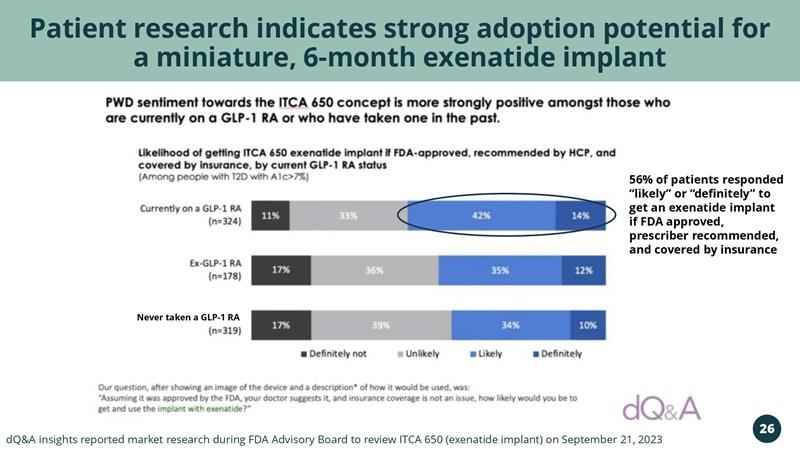
Patient research indicates strong adoption potential for a miniature, 6-month exenatide implant
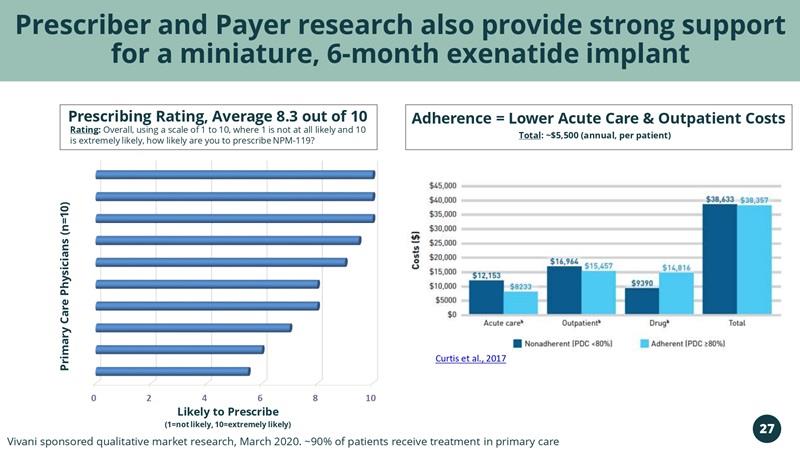
Prescriber and Payer research also provide strong support for a miniature, 6-month exenatide implant
NPM-119 Clinical + Regulatory Development Near-Term Plan


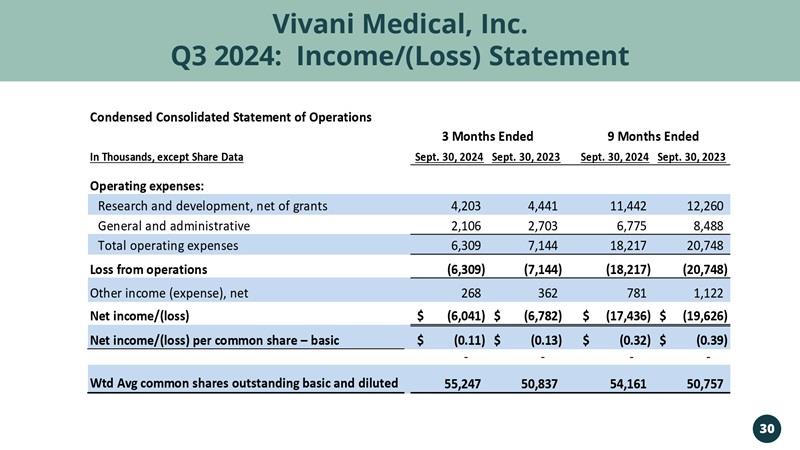
Vivani Medical, Inc. Q3 2024: Income/(Loss) Statement
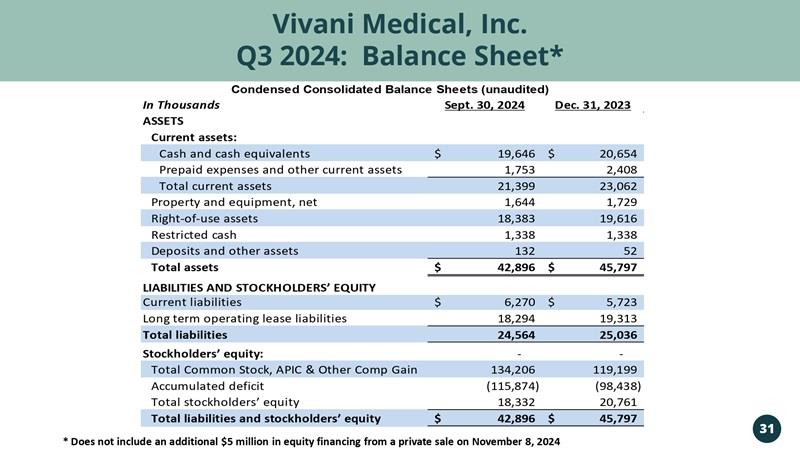
Vivani Medical, Inc. Q3 2024: Balance Sheet*
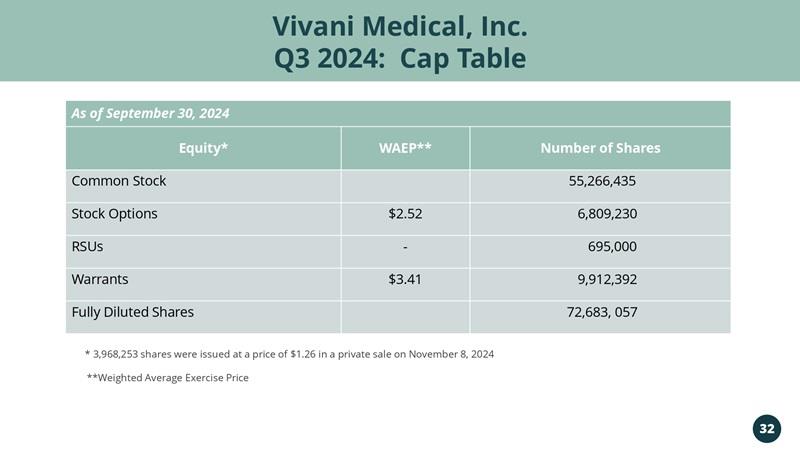
Vivani Medical, Inc. Q3 2024: Cap Table
An innovative, biopharmaceutical company developing a portfolio of ultra long-acting, miniature, drug implants to treat chronic diseases. NanoPortal™ platform technology enables the design of implants aimed at improving medication non-adherence and tolerability.