Vivani Medical Announces Positive NPM-115 Preclinical Weight Loss Data Comparable to Ozempic®/Wegovy® and Discloses NPM-139 as Semaglutide as Strategy Shifts to Prioritize Obesity Portfolio
NPM-115 (exenatide implant) generated significant weight loss comparable to injectable semaglutide (Ozempic®/Wegovy®) from a single administration with expected twice-yearly dosing
Vivani discloses semaglutide as the active pharmaceutical ingredient in NPM-139, with the added potential benefit of once-yearly dosing
NPM-115 and NPM-139 are miniature, subdermal implants in development for chronic weight management designed to guarantee medication adherence and potentially improve treatment tolerability by providing smooth and steady delivery of GLP-1 therapy
ALAMEDA, Calif.--(BUSINESS WIRE)-- Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative, preclinical-stage biopharmaceutical company developing novel, long-term drug implants, today announced positive preclinical data on weight loss effects for NPM-115, the Company’s miniature, twice-yearly, exenatide implant under development for the treatment of chronic weight management. The Company also disclosed that semaglutide is the active pharmaceutical ingredient in NPM-139, a miniature, subdermal GLP-1 implant in development for chronic weight management, with the added potential benefit of once-yearly administration. These developments are part of a strategic shift to prioritize the Company’s obesity implants based on emerging data regarding the potential for high-dose GLP-1 products to improve health outcomes for obese and overweight patients.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240228342864/en/
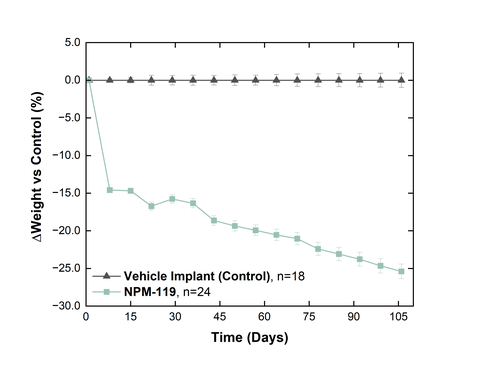
Weight difference from control in healthy Sprague-Dawley Rats. % weight change from baseline for NPM-119 (exenatide) corrected to control (vehicle implant). Values are mean ± SE. (Photo: Business Wire)
“In response to tremendous medical need and unprecedented market demand, we are prioritizing the development of our GLP-1 implants for the treatment of obesity and chronic weight management. Since a high-dose GLP-1 implant for obesity would likely also be able to address our previous type 2 diabetes focus, the recently generated compelling weight loss data from NPM-115 naturally supports a shift in focus towards an indication with even broader potential. We believe the primary expected advantages of our proprietary NanoPortal™ implant technology, improving medication adherence and medication tolerability, have the potential to transform and advance the adoption of GLP-1 therapy in the future.” said Adam Mendelsohn, Ph.D., Vivani President and Chief Executive Officer. “The potential for long-term GLP-1 implants becomes even more compelling when you consider that the improved adherence and persistence of Ozempic and Wegovy over prior obesity medications is still only 40% as recently reported in a large retrospective cohort study published in the research journal Obesity. Collectively, the potential for improvement in medication adherence, tolerability and real-world patient outcomes motivates us to rapidly advance the development of NPM-115, NPM-139 and the balance of our portfolio.”
In a study in high-fat diet-induced obese mice, NPM-115 generated weight loss of approximately 20% compared to a sham implant control after a 28-day treatment duration, comparable to weight loss observed in mice treated with semaglutide injections (Ozempic/Wegovy) in the same study. The supratherapeutic doses provided for both NPM-115 (single administration delivering exenatide at ~530 nmol/kg/day), and semaglutide (weekly injections of ~2,700 nmol/kg/week), were selected to maximize the weight-loss potential of both exenatide and semaglutide.
In a second study in healthy rats, a single administration of the Company’s exenatide implant NPM-119, in development for the treatment of type 2 diabetes, resulted in body weights that were approximately 25% lower than a vehicle implant control after 15 weeks of treatment with an expected duration of effect of six months. NPM-119 delivered exenatide at a rate of approximately 320 nmol/kg/day and has demonstrated smooth, non-fluctuating release of exenatide in both in vitro and in vivo studies. NPM-119 has previously demonstrated pharmacokinetic data exhibiting continuous and therapeutic exenatide exposure levels over a six-month duration in healthy rats. Since NPM-115 is a higher-dose version of an otherwise similar product as NPM-119, the durability of the effect on weight demonstrated in this study is expected to translate to future studies utilizing NPM-115.
These preclinical data provide further evidence that the weight loss potential of exenatide treatment in humans may be comparable to other GLP-1 molecules such as semaglutide assuming adequate exposure levels are achieved and maintained. The weight loss potential of exenatide in humans has not been fully evaluated in the currently marketed exenatide products Byetta® (twice-daily injection) and Bydureon® (weekly injection) potentially due to limitations associated with adherence and dosing. NPM-115 directly addresses these limitations. It is designed to improve adherence by enabling patients to receive continuous dosing over a six-month interval from a single administration. NPM-115 is planned to maximize exenatide’s weight loss effect in humans, pending further development and regulatory clearance, by evaluating exenatide exposure levels higher than previously explored.
Dr. Mendelsohn will present study results on May 17 at the TIDES USA 2024 conference in Boston.
About Vivani Medical, Inc.
Leveraging its proprietary NanoPortal™ platform, Vivani Medical develops biopharmaceutical implants designed to deliver drug molecules steadily over extended periods of time with the goal of guaranteeing adherence, and potentially to improve medication tolerability. Vivani’s NPM-115 and NPM-119 are miniature, six-month, GLP-1 implants in development for the treatment of chronic weight management in obese or overweight patients and type 2 diabetes, respectively. Both NPM-115 and NPM-119 are exenatide based products with a higher-dose associated with NPM-115 for the treatment of chronic weight management in obese or overweight patients. An IND for NPM-119’s first-in-human study LIBERATE-1 has been submitted and is on clinical hold pending requests by the FDA for additional chemistry, manufacturing, and controls (CMC) information. Vivani anticipates submitting the requested CMC information to the FDA in the first half of 2024. LIBERATE-1 is a randomized, 12-week investigation of the safety, tolerability, and full pharmacokinetic profile of NPM-119 in patients with type 2 diabetes. Vivani is also preparing to submit an IND for a first-in-human study with NPM-115 for the treatment of chronic weight management later this year. These NanoPortalTM implants are designed to provide patients with the opportunity to realize the full potential benefit of their medication by avoiding the challenges associated with the daily or weekly administration of orals and injectables. Medication non-adherence occurs when patients do not take their medication as prescribed. This affects an alarming number of patients, approximately 50%, including those taking daily pills. Medication non-adherence, which contributes to more than $500 billion in annual avoidable healthcare costs and 125,000 potentially preventable deaths annually in the U.S. alone, is a primary and daunting reason why obese or overweight patients, and patients taking type 2 diabetes or other chronic disease medications face significant challenges in achieving positive real-world effectiveness.
Vivani’s wholly owned subsidiary Cortigent is developing targeted neurostimulation systems intended to help patients recover critical body functions. Investigational devices include Orion®, designed to provide artificial vision to people who are profoundly blind, and a new system intended to accelerate the recovery of arm and hand function in patients who are partially paralyzed due to stroke. Vivani continues to assess strategic options for advancing Cortigent’s pioneering technology.
Forward-Looking Statements
This press release contains certain “forward-looking statements” within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: “target,” “believe,” “expect,” “will,” “may,” “anticipate,” “estimate,” “would,” “positioned,” “future,” and other similar expressions that in this press release, including statements regarding our business, products in development, including the therapeutic potential thereof, plans to address any requests from the FDA related to the agency’s current clinical hold on NPM-119, the initiation of the LIBERATE-1 trial and reporting of trial results, the planned development therefor, our emerging development plans for NPM-115, NPM-139, or our plans with respect to Cortigent and its proposed initial public offering, technology, strategy, cash position and financial runway. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations, and assumptions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Actual results and outcomes may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause actual results and outcomes to differ materially from those indicated in the forward-looking statements include, among others, risks related to the development and commercialization of our products, including NPM-115 and NPM-119; delays and changes in the development of our products, including our ability to address any requests from the FDA related to LIBERATE-1 and to commence clinical development of NPM-119, including as a result of applicable laws, regulations and guidelines, potential delays in submitting and receiving regulatory clearance or approval to conduct our development activities, risks related to the initiation, enrollment and conduct of our planned clinical trials and the results therefrom; our history of losses and our ability to access additional capital or otherwise fund our business; market conditions and the ability of Cortigent to complete its initial public offering. There may be additional risks that the Company considers immaterial, or which are unknown. A further list and description of risks and uncertainties can be found in the Company’s most recent Annual Report on Form 10-K filed with the SEC filed on March 31, 2023, as updated by our subsequent Quarterly Reports on Form 10-Q. Any forward-looking statement made by us in this press release is based only on information currently available to the Company and speaks only as of the date on which it is made. The Company undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of added information, future developments or otherwise, except as required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240228342864/en/
Company Contact:
Donald Dwyer
Chief Business Officer
info@vivani.com
(415) 506-8462
Investor Relations Contact:
Brigid A. Makes
Chief Financial Officer
investors@vivani.com
(415) 506-8462
Media Contact:
Sean Leous
ICR Westwicke
Sean.Leous@westwicke.com
(646) 866-4012
Source: Vivani Medical, Inc.
Released February 28, 2024